Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation - PubMed (original) (raw)
Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation
Bradley W McDill et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Congenital progressive hydronephrosis (cph) is a spontaneous recessive mutation that causes severe hydronephrosis and obstructive nephropathy in affected mice. The mutation has been mapped to the distal end of mouse chromosome 15, but the mutated gene has not been found. Here, we describe the identification of a single base pair change in aquaporin-2 (Aqp2) in cph mutants through genetic linkage mapping. The C-T change led to the substitution of a Ser (S256) by a Leu in the cytoplasmic tail of the Aqp2 protein, preventing its phosphorylation at S256 and the subsequent accumulation of Aqp2 on the apical membrane of the collecting duct principal cells. The interference with normal trafficking of Aqp2 by this mutation resulted in a severe urine concentration defect. cph homozygotes demonstrated polydipsia and produced a copious amount of hypotonic urine. The urine concentration defect could not be corrected by [deamino-Cys1,D-Arg8]-vasopressin (DDAVP, a vasopressin analog), characteristic of nephrogenic diabetes insipidus. The nephrogenic diabetes insipidus symptoms and the absence of developmental defects in the pyeloureteral peristaltic machinery in the mutants before the onset of hydronephrosis suggest that the congenital obstructive nephropathy is most likely a result of the polyuria. This study has revealed the genetic basis for the classical cph mutation and has provided direct genetic evidence that S256 in Aqp2 is indispensable for the apical accumulation, but not the general glycosylation or membrane association, of Aqp2.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
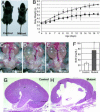
The cph mutants have apparent congenital obstruction at multiple levels. (A) At 2 weeks of age, the mutants are significantly smaller than their control littermates. (Scale bar: 1 cm.) (B) The mutants grow slower than the controls. ▴, controls; ■, mutants. (C_–_E) The mutants have unilateral or bilateral hydronephrosis and hydroureter (arrow). (F) The blood urea nitrogen (BUN) level in the mutants (P5–P16) is 70.0 ± 25.0 mg/dl (n = 35), significantly (P < 0.001) higher than that of the controls (P5–P16, 26.8 ± 5.0 mg/dl; n = 29). (G and H) Kidney sections from P14 control and mutant littermates stained with hematoxylin/eosin.
Fig. 2.
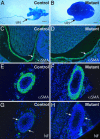
The cph mutants do not have complete physical obstruction or gross developmental abnormalities in the smooth muscles and nerves along the urinary tract. (A and B) Resin moldings of the pelvicocaliceal space and ureteric lumen at P5. (Scale bars: 1 mm.) (C_–_F) αSMA staining (green) of the smooth muscles in the pelvic wall (arrows in C and D) and ureteric sections (E and F) at P6. (G and H) Nerve fibers (arrows) on the cross sections of the ureters from P6 mice were revealed by the antineurofilament antibody (NF, green). The slides were counterstained with DAPI to reveal the nuclei (blue).
Fig. 3.
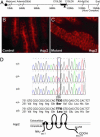
Genetic linkage mapping and positional cloning of cph. (A) Physical map of relevant portions of mouse chromosome 15. Our genetic mapping efforts locate the cph locus to the chromosomal interval of ≈0.7 Mbp, defined by the microsatellite markers C15LD6 and C15LD5. The black triangle indicates the chromosomal location of Aqp2. (B and C) Immunostaining of the collecting ducts in the outer medulla shows the apical accumulation of Aqp2 in the controls (B) but a diffuse staining with no apical accumulation in the mutant (C). (D) Sequence of the fourth exon of Aqp2 reveals the C-T substitution at nucleotide 767 in the homozygous mutants, whereas the heterozygotes have both C and T represented at position 767. This substitution results in a Ser to Leu change at amino acid 256 in the cytoplasmic tail of the Aqp2 protein. +, WT allele; c, cph mutant allele.
Fig. 4.
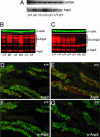
Absence of S256 phosphorylation and increased Aqp2 expression in the cph mutants. (A) RT-PCR of total RNA from whole kidneys revealed an increase of Aqp2 transcripts in the mutants (c/c). (B) Western blot of total Aqp2 protein from control and mutant kidneys. G-Aqp2 indicates the various forms of glycosylated Aqp2. ∗, a band that cross-reacts with the secondary antibody alone and is deemed unrelated to Aqp2. Aqp2 protein levels in the mutant samples are elevated. (C) Western blot of proteins from whole kidney extracts probed with an Aqp2-S256 phosphorylation-specific antibody. The mutant samples (c/c) lost the S256 phosphorylation on Aqp2. (D_–_G) Immunostaining of the outer medullary collecting ducts. The Aqp2-S256 phospho-specific antibody (pAqp2) revealed apical distribution of the S256-phosphorylated Aqp2 (red) in the WT principal cells labeled by Dolichos biflorus agglutinin (green) (F), closely resembling the Aqp2 distribution shown by the nonphospho-specific Aqp2 antibody staining (red) on adjacent sections (D). The mutant (c/c), however, has a complete absence of phospho-specific Aqp2 staining (G), but apparently overexpresses the Aqp2-S256L that lacks the S256 phosphorylation (E).
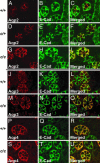
Fig. 5.
Aqp2-S256L has membrane association but lacks apical accumulation. Paraffin sections of the outer medulla from P1 mice of WT (A_–_C), heterozygous (D_–_F), and homozygous (G_–_I) littermates were stained with an Aqp2 antibody (A, D, and G) or an anti-E-Cadherin (E-Cad) antibody (B, E, and H). C, F, and I are merged images of the first two channels. E-Cadherin is expressed on the basolateral membrane whereas Aqp2 accumulates on the apical membrane of the principal cells in the WT. There is very little overlap between the Aqp2 staining and the E-Cadherin staining. In the cph homozygotes, however, Aqp2-S256L does not accumulate on the apical membrane but shows a prominent basolateral distribution as revealed by the yellow signal produced by the overlapping red Aqp2 and green E-Cadherin staining on basolateral membranes. Although the overall pattern of Aqp2 expression in the heterozygotes is similar to that of the WT mice, the heterozygotes show a higher degree of basolateral distribution of Aqp2 compared with the WT (A_–_I). In cph mutants, both Aqp3 and Aqp4 are expressed on the basolateral membrane and at levels similar to those seen in the WT mice (J_–_U).
Fig. 6.
The obstructive nephropathy is likely induced by polyuria in the cph mutants. (A) cph mutants have defects in urine concentration. The urine osmolality in adult mice is significantly lower in the cph homozygotes (480.8 ± 258.0; n = 10) versus WT (2535.9 ± 283.6; n = 8) and heterozygotes (2192.3 ± 289.8; n = 16). ∗, P < 0.001 compared with the other two groups. All measurements are in mOsm/kg unless otherwise stated. (B) Serum osmolality is significantly increased in the homozygotes (391.9 ± 76.7; n = 27) compared with the WT (278.8 ± 6.7; n = 6) and heterozygous mice (287.6 ± 16.4; n = 20). ∗, P < 0.001 compared with the other two groups. (C) cph homozygotes have increased water input (21.6 ± 9.4 ml; n = 11) and urine output (15.8 ± 8.1 ml) than those of the WT (3.7 ± 0.9 ml water intake and 1.1 ± 0.8 ml urine output, n = 9) and heterozygous (4.0 ± 1.1 ml of water intake and 1.2 ± 0.6 ml of urine output; n = 11) littermates. ∗, P < 0.001 compared with the other two groups. (D) DDAVP injection promotes urine concentration in the WT and heterozygotes but not in the cph homozygotes. The postinjection urine osmolality is increased from 2,385.3 ± 265.7 (n = 4) and 2,127.3 ± 228.2 (n = 11) to 3,030.0 ± 554.7 and 2,571.4 ± 343.3 for +/+ and c/+ mice respectively. ∗, P < 0.05 compared with measurements taken before injection. The osmolality of the post injection urine of the mutant is not significantly different from that measured before the injection (from 614.7 ± 222.5 to 584.3 ± 260.3; n = 11). Bfr, before DDAVP injection; Aftr, after DDAVP injection.
Similar articles
- p.R254Q mutation in the aquaporin-2 water channel causing dominant nephrogenic diabetes insipidus is due to a lack of arginine vasopressin-induced phosphorylation.
Savelkoul PJ, De Mattia F, Li Y, Kamsteeg EJ, Konings IB, van der Sluijs P, Deen PM. Savelkoul PJ, et al. Hum Mutat. 2009 Oct;30(10):E891-903. doi: 10.1002/humu.21082. Hum Mutat. 2009. PMID: 19585583 - Nephrogenic diabetes insipidus in mice caused by deleting COOH-terminal tail of aquaporin-2.
Shi PP, Cao XR, Qu J, Volk KA, Kirby P, Williamson RA, Stokes JB, Yang B. Shi PP, et al. Am J Physiol Renal Physiol. 2007 May;292(5):F1334-44. doi: 10.1152/ajprenal.00308.2006. Epub 2007 Jan 16. Am J Physiol Renal Physiol. 2007. PMID: 17229678 Free PMC article. - Lack of arginine vasopressin-induced phosphorylation of aquaporin-2 mutant AQP2-R254L explains dominant nephrogenic diabetes insipidus.
de Mattia F, Savelkoul PJ, Kamsteeg EJ, Konings IB, van der Sluijs P, Mallmann R, Oksche A, Deen PM. de Mattia F, et al. J Am Soc Nephrol. 2005 Oct;16(10):2872-80. doi: 10.1681/ASN.2005010104. Epub 2005 Aug 24. J Am Soc Nephrol. 2005. PMID: 16120822 - Hereditary Nephrogenic Diabetes Insipidus: Pathophysiology and Possible Treatment. An Update.
Milano S, Carmosino M, Gerbino A, Svelto M, Procino G. Milano S, et al. Int J Mol Sci. 2017 Nov 10;18(11):2385. doi: 10.3390/ijms18112385. Int J Mol Sci. 2017. PMID: 29125546 Free PMC article. Review.
Cited by
- A new tool for conditional gene manipulation in a subset of keratin-expressing epithelia.
Wang Y, Guo Q, Casey A, Lin C, Chen F. Wang Y, et al. Genesis. 2012 Dec;50(12):899-907. doi: 10.1002/dvg.22046. Epub 2012 Aug 20. Genesis. 2012. PMID: 22764128 Free PMC article. - Adam10 mediates the choice between principal cells and intercalated cells in the kidney.
Guo Q, Wang Y, Tripathi P, Manda KR, Mukherjee M, Chaklader M, Austin PF, Surendran K, Chen F. Guo Q, et al. J Am Soc Nephrol. 2015 Jan;26(1):149-59. doi: 10.1681/ASN.2013070764. Epub 2014 Jun 5. J Am Soc Nephrol. 2015. PMID: 24904084 Free PMC article. - Lower urinary tract development and disease.
Rasouly HM, Lu W. Rasouly HM, et al. Wiley Interdiscip Rev Syst Biol Med. 2013 May-Jun;5(3):307-42. doi: 10.1002/wsbm.1212. Epub 2013 Feb 13. Wiley Interdiscip Rev Syst Biol Med. 2013. PMID: 23408557 Free PMC article. Review. - Activation of NFAT signaling establishes a tumorigenic microenvironment through cell autonomous and non-cell autonomous mechanisms.
Tripathi P, Wang Y, Coussens M, Manda KR, Casey AM, Lin C, Poyo E, Pfeifer JD, Basappa N, Bates CM, Ma L, Zhang H, Pan M, Ding L, Chen F. Tripathi P, et al. Oncogene. 2014 Apr 3;33(14):1840-9. doi: 10.1038/onc.2013.132. Epub 2013 Apr 29. Oncogene. 2014. PMID: 23624921 Free PMC article. - Absence of canonical Smad signaling in ureteral and bladder mesenchyme causes ureteropelvic junction obstruction.
Tripathi P, Wang Y, Casey AM, Chen F. Tripathi P, et al. J Am Soc Nephrol. 2012 Apr;23(4):618-28. doi: 10.1681/ASN.2011060566. Epub 2012 Jan 26. J Am Soc Nephrol. 2012. PMID: 22282597 Free PMC article.
References
- Chevalier R. L. Pediatr. Nephrol. 1999;13:612–619. - PubMed
- Grasso M., Gitlin J. eMedicine. 2001. www.emedicine.com/med/topic3074.htm.
- Chertin B., Puri P. J. Urol. 2003;169:1804–1808. - PubMed
- Peters C. A. Prenatal Diagn. 2001;21:917–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK067386/DK/NIDDK NIH HHS/United States
- R21 DK064816/DK/NIDDK NIH HHS/United States
- R01DK067386/DK/NIDDK NIH HHS/United States
- R21DK64816/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous