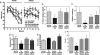Reversal of Alzheimer's-like pathology and behavior in human APP transgenic mice by mutation of Asp664 - PubMed (original) (raw)
. 2006 May 2;103(18):7130-5.
doi: 10.1073/pnas.0509695103. Epub 2006 Apr 25.
Olivia F Gorostiza, Surita Banwait, Marina Ataie, Anna V Logvinova, Sandhya Sitaraman, Elaine Carlson, Sarah A Sagi, Nathalie Chevallier, Kunlin Jin, David A Greenberg, Dale E Bredesen
Affiliations
- PMID: 16641106
- PMCID: PMC1459029
- DOI: 10.1073/pnas.0509695103
Reversal of Alzheimer's-like pathology and behavior in human APP transgenic mice by mutation of Asp664
Veronica Galvan et al. Proc Natl Acad Sci U S A. 2006.
Erratum in
- Proc Natl Acad Sci U S A. 2007 Apr 17;104(16):6876
Abstract
The deficits characteristic of Alzheimer's disease (AD) are believed to result, at least in part, from the neurotoxic effects of beta-amyloid peptides, a set of 39-43 amino acid fragments derived proteolytically from beta-amyloid precursor protein (APP). APP also is cleaved intracytoplasmically at Asp-664 to generate a second cytotoxic peptide, APP-C31, but whether this C-terminal processing of APP plays a role in the pathogenesis of AD is unknown. Therefore, we compared elements of the Alzheimer's phenotype in transgenic mice modeling AD with vs. without a functional Asp-664 caspase cleavage site. Surprisingly, whereas beta-amyloid production and plaque formation were unaltered, synaptic loss, astrogliosis, dentate gyral atrophy, increased neuronal precursor proliferation, and behavioral abnormalities were completely prevented by a mutation at Asp-664. These results suggest that Asp-664 plays a critical role in the generation of Alzheimer-related pathophysiological and behavioral changes in human APP transgenic mice, possibly as a cleavage site or via protein-protein interactions.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1
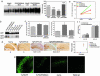
Characterization of PDAPP and PDAPP(D664A) mice. (a) APP expression. (a Left) Human and mouse APP were detected in brain homogenates by using the anti-APP CT15 antibody. (a Right) Densitometric quantitation of immunoreactivity. (b) Detection of soluble Aβ peptide. Aβ peptides in 3- to 4-mo transgenic mouse brains were detected by immunoprecipitation, followed by Western blotting with 26D6 antibody. [Note that, although Western blots suggested similar levels of expression of Aβ1–40 and Aβ1–42 by PDAPP(J20) and PDAPP(D664A)(B21), ELISA quantitations (Fig. 1_c_) reproducibly demonstrated that expression by PDAPP(J20) was greater than that of PDAPP(D664A)(B21)]. (c) Quantitation of soluble Aβ. Aβ1–40 and Aβ1–42 were determined at 3–4 months by ELISA as described in Methods (n = 26). (d) Quantitation of Aβ deposits. (d Left) Fifty-micrometer vibratome brain sections of transgenic 12-mo mice were stained with 3D6 antibody. (d Right) Total hippocampal Aβ plaques were counted by investigators blinded to strain and genotype (n = 18); means ± SEM. (e and f) Quantitation of soluble Aβ. ELISA assays were as described in Methods. (g) Cleavage of APP at Asp-664 in vivo. An antibody specific for the neoepitope generated by cleavage of APP at Asp-664 (refs. and ; see also Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site) was used to demonstrate an increase in cleavage in PDAPP in comparison with both controls and PDAPP(D664A) mice.
Fig. 2
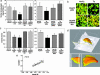
Effect of D664A mutation on synaptic loss and dentate atrophy. (a) Quantitation of presynaptic densities. (a Left) Quantitation of presynaptic densities in brains of 8- to 10-mo mice as described in Methods (n = 48). (a Right) Quantitation of presynaptic densities in sections from brains of 5-mo mice (n = 16). {Note that, because of dynamic range and total fluorescence variability from experiment to experiment, PDAPP(J20) results from early experiments [vs. PDAPP(D664A)(B21)] and later studies [vs. PDAPP(D664A)(B254)] were not pooled.} (b) CA1 stratum radiatum in hippocampal sections stained with α-synaptophysin antibodies. (c) Volume determinations. (c Left) DG volumes were determined by using
imaris
3D and confirmed by Cavalieri analysis as described in Methods (n = 38). Cavalieri results are shown. (c Right)
imaris
3D comparisons of J20 and B254. (d) Orthogonal, saggital, and coronal views of 3D surface reconstructions of DG molecular layers of representative PDAPP(J20) (red) and PDAPP(D664A)(B21) (yellow) mice. (e) Volumes derived by Cavalieri analysis and
imaris
3D reconstructions were highly correlated (_r_2 = 0.72; P < 0.00001; n = 28). No significant difference was found in body or brain weight between strains or genotypes. Samples were coded to blind investigators with respect to strain and genotype. Data are expressed as mean ± SEM. ∗∗, significance (P < 0.05) was determined by ANOVA followed by the Kruskal–Wallis test. The Pearson correlation coefficient test, followed by the runs test, was used for regression analyses.
Fig. 3
Effect of the D664A mutation on astrogliosis. Sections from 12-mo animals were stained with anti-GFAP antibodies (a) and total GFAP-immunopositive areas were determined (b) as described in Methods (n = 6); means ± SEM. ∗, P < 0.05 by ANOVA followed by the Kruskal–Wallis test.
Fig. 4
Effect of the D664A mutation on behavior in PDAPP mice. (a and b) Morris water maze. (a) Learning curves. Mean latencies on 6 consecutive days of training (average of 4–6 trials per day ± SEM). Repeated-measures ANOVA revealed that all groups learned the cued task [F(11, 330) = 21.56, P < 0.0001, visible]. PDAPP(J20) animals (n = 8) showed deficits during acquisition in the hidden, hippocampal-dependent component of the task. ∗, significant difference from nontransgenic PDAPP(J20) (n = 6), nontransgenic PDAPP(D664A)(B21) (n = 10), and transgenic PDAPP(D664A)(B21) animals (n = 10) [F (3, 180) = 7.16; P < 0.0001, two-way ANOVA]. (b) Day 9 probe trial. Percentage of time spent in the target quadrant during the probe trial (corrected for thigmotaxis). ∗, significant difference from nontransgenic PDAPP(J20); P < 0.05. No significant difference in the time spent in the target quadrant was observed between PDAPP(D664A)(B21) transgenic and nontransgenic animals. (c) Day 9 probe trial. Number of target crossings during the posttraining probe trial. ∗, significant difference from nontransgenic PDAPP(J20); P < 0.02 by student's t test; means ± SEM are shown. (d) Spontaneous alternation in the Y maze. Spontaneous alternation was significantly reduced in PDAPP(J20) transgenics (P < 0.05; Tukey's post hoc test applied to a significant effect of genotype in ANOVA; n = 38). The dotted line shows chance levels of performance. (e) Spontaneous activity in the Y maze. B254 transgenic animals demonstrated an increase in spontaneous activity. (f) Novel object exploration. Transgenic PDAPP(J20) animals spent significantly less time exploring a novel object (nonrelated pup) in an 8-min period (Tukey's post hoc test applied to a significant effect of genotype in ANOVA; P < 0.01) than all other groups (n = 38).
Similar articles
- Long-term prevention of Alzheimer's disease-like behavioral deficits in PDAPP mice carrying a mutation in Asp664.
Galvan V, Zhang J, Gorostiza OF, Banwait S, Huang W, Ataie M, Tang H, Bredesen DE. Galvan V, et al. Behav Brain Res. 2008 Aug 22;191(2):246-55. doi: 10.1016/j.bbr.2008.03.035. Epub 2008 Apr 8. Behav Brain Res. 2008. PMID: 18485495 Free PMC article. - Signal transduction in Alzheimer disease: p21-activated kinase signaling requires C-terminal cleavage of APP at Asp664.
Nguyen TV, Galvan V, Huang W, Banwait S, Tang H, Zhang J, Bredesen DE. Nguyen TV, et al. J Neurochem. 2008 Feb;104(4):1065-80. doi: 10.1111/j.1471-4159.2007.05031.x. Epub 2007 Nov 6. J Neurochem. 2008. PMID: 17986220 Free PMC article. - Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice.
Dewachter I, Reversé D, Caluwaerts N, Ris L, Kuipéri C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. Dewachter I, et al. J Neurosci. 2002 May 1;22(9):3445-53. doi: 10.1523/JNEUROSCI.22-09-03445.2002. J Neurosci. 2002. PMID: 11978821 Free PMC article. - C-terminal cleavage of the amyloid-beta protein precursor at Asp664: a switch associated with Alzheimer's disease.
Banwait S, Galvan V, Zhang J, Gorostiza OF, Ataie M, Huang W, Crippen D, Koo EH, Bredesen DE. Banwait S, et al. J Alzheimers Dis. 2008 Feb;13(1):1-16. doi: 10.3233/jad-2008-13101. J Alzheimers Dis. 2008. PMID: 18334752 Free PMC article. - Enriched endogenous n-3 polyunsaturated fatty acids alleviate cognitive and behavioral deficits in a mice model of Alzheimer's disease.
Wu K, Gao X, Shi B, Chen S, Zhou X, Li Z, Gan Y, Cui L, Kang JX, Li W, Huang R. Wu K, et al. Neuroscience. 2016 Oct 1;333:345-55. doi: 10.1016/j.neuroscience.2016.07.038. Epub 2016 Jul 27. Neuroscience. 2016. PMID: 27474225
Cited by
- Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain.
Ghosal K, Vogt DL, Liang M, Shen Y, Lamb BT, Pimplikar SW. Ghosal K, et al. Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18367-72. doi: 10.1073/pnas.0907652106. Epub 2009 Oct 16. Proc Natl Acad Sci U S A. 2009. PMID: 19837693 Free PMC article. - Genetic and pharmacologic proteasome augmentation ameliorates Alzheimer's-like pathology in mouse and fly APP overexpression models.
Chocron ES, Munkácsy E, Kim HS, Karpowicz P, Jiang N, Van Skike CE, DeRosa N, Banh AQ, Palavicini JP, Wityk P, Kalinowski L, Galvan V, Osmulski PA, Jankowska E, Gaczynska M, Pickering AM. Chocron ES, et al. Sci Adv. 2022 Jun 10;8(23):eabk2252. doi: 10.1126/sciadv.abk2252. Epub 2022 Jun 8. Sci Adv. 2022. PMID: 35675410 Free PMC article. - Alzheimer's disease: ageing-related or age-related? New hypotheses from an old debate.
Bugiani O. Bugiani O. Neurol Sci. 2011 Dec;32(6):1241-7. doi: 10.1007/s10072-011-0614-4. Epub 2011 May 13. Neurol Sci. 2011. PMID: 21567180 - A tunable, modular approach to fluorescent protease-activated reporters.
Wu P, Nicholls SB, Hardy JA. Wu P, et al. Biophys J. 2013 Apr 2;104(7):1605-14. doi: 10.1016/j.bpj.2013.01.058. Biophys J. 2013. PMID: 23561537 Free PMC article. - Next generation therapeutics for Alzheimer's disease.
Bredesen DE, John V. Bredesen DE, et al. EMBO Mol Med. 2013 Jun;5(6):795-8. doi: 10.1002/emmm.201202307. Epub 2013 May 23. EMBO Mol Med. 2013. PMID: 23703924 Free PMC article.
References
- Selkoe D. J. Science. 2002;298:789–791. - PubMed
- Gervais F. G., Xu D., Robertson G. S., Vaillancourt J. P., Zhu Y., Huang J., LeBlanc A., Smith D., Rigby M., Shearman M. S., et al. Cell. 1999;97:395–406. - PubMed
- Lu D. C., Rabizadeh S., Chandra S., Shayya R. F., Ellerby L. M., Ye X., Salvesen G. S., Koo E. H., Bredesen D. E. Nat. Med. 2000;6:397–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases