Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses - PubMed (original) (raw)
Comparative Study
Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses
Friedemann Weber et al. J Virol. 2006 May.
Abstract
Double-stranded RNA (dsRNA) longer than 30 bp is a key activator of the innate immune response against viral infections. It is widely assumed that the generation of dsRNA during genome replication is a trait shared by all viruses. However, to our knowledge, no study exists in which the production of dsRNA by different viruses is systematically investigated. Here, we investigated the presence and localization of dsRNA in cells infected with a range of viruses, employing a dsRNA-specific antibody for immunofluorescence analysis. Our data revealed that, as predicted, significant amounts of dsRNA can be detected for viruses with a genome consisting of positive-strand RNA, dsRNA, or DNA. Surprisingly, however, no dsRNA signals were detected for negative-strand RNA viruses. Thus, dsRNA is indeed a general feature of most virus groups, but negative-strand RNA viruses appear to be an exception to that rule.
Figures
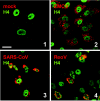
FIG. 1.
The monoclonal antibody J2 specifically recognizes dsRNA. Vero cells were transfected with poly(I:C) as indicated in Materials and Methods. After an incubation period of 6 h, the cells were fixed and analyzed for dsRNA using the mouse monoclonal antibody J2 (red). To visualize the cell nuclei, histone H4 was stained using a specific rabbit antiserum (green). Shown are untransfected cells (1), poly(I:C)-transfected cells (2), and poly(I:C)-transfected cells treated with either 2 U of RNase III (3) or 2 U of RNase A (4). Bar, 20 μm. All pictures were taken with the same magnification.
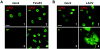
FIG. 2.
dsRNA in cells infected with positive-strand RNA and dsRNA viruses. Vero cells were infected at a multiplicity of infection of 5 with EMCV (2), SARS-CoV (3), or ReoV (4) or left uninfected (1). At 5 h (EMCV), 16 h (SARS-CoV), or 48 h (ReoV) postinfection, cells were fixed and analyzed by immunofluorescence as indicated in the legend to Fig. 1. Bar, 20 μm. All pictures were taken with the same magnification. The differences in size and morphology of the nuclei are most probably caused by initiation of apoptosis or by disturbances in nuclear-cytoplasmic transport, as has been described for several viruses (6, 21, 23, 27).
FIG. 3.
dsRNA in cells infected with DNA viruses. Vero cells were infected at a multiplicity of infection of 5 with AdV (2), HSV (3), or Vac (4) or left uninfected (1). At 7 h (AdV, HSV) or 5 h (Vac) postinfection, cells were fixed and analyzed by immunofluorescence as indicated in the legend to Fig. 1. Bar, 20 μm. All pictures were taken with the same magnification.
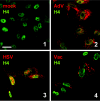
FIG. 4.
Negative-strand RNA viruses. Vero cells were infected at a multiplicity of infection of 5 with FLUAV (A, panels 2 and 4) or LACV (B, panels 2 and 4) or left uninfected (A and B, panels 1 and 3). At 5 h postinfection, cells were fixed and analyzed by immunofluorescence either for dsRNA as indicated in the legend to Fig. 1 (A and B, panels 1 and 2) or for viral antigens (A and B, panels 3 and 4). Bar, 20 μm. All pictures were taken with the same magnification.
Similar articles
- Double-Stranded RNA Is Detected by Immunofluorescence Analysis in RNA and DNA Virus Infections, Including Those by Negative-Stranded RNA Viruses.
Son KN, Liang Z, Lipton HL. Son KN, et al. J Virol. 2015 Sep;89(18):9383-92. doi: 10.1128/JVI.01299-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136565 Free PMC article. - dsRNA-Seq: Identification of Viral Infection by Purifying and Sequencing dsRNA.
Decker CJ, Steiner HR, Hoon-Hanks LL, Morrison JH, Haist KC, Stabell AC, Poeschla EM, Morrison TE, Stenglein MD, Sawyer SL, Parker R. Decker CJ, et al. Viruses. 2019 Oct 14;11(10):943. doi: 10.3390/v11100943. Viruses. 2019. PMID: 31615058 Free PMC article. - Inhibition of viruses by RNA interference.
Stram Y, Kuzntzova L. Stram Y, et al. Virus Genes. 2006 Jun;32(3):299-306. doi: 10.1007/s11262-005-6914-0. Virus Genes. 2006. PMID: 16732482 Free PMC article. Review. - A Novel Mechanism Underlying the Innate Immune Response Induction upon Viral-Dependent Replication of Host Cell mRNA: A Mistake of +sRNA Viruses' Replicases.
Delgui LR, Colombo MI. Delgui LR, et al. Front Cell Infect Microbiol. 2017 Jan 20;7:5. doi: 10.3389/fcimb.2017.00005. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28164038 Free PMC article. Review.
Cited by
- Systematic genetic characterization of the human PKR kinase domain highlights its functional malleability to escape a poxvirus substrate mimic.
Chambers MJ, Scobell SB, Sadhu MJ. Chambers MJ, et al. Elife. 2024 Nov 12;13:RP99575. doi: 10.7554/eLife.99575. Elife. 2024. PMID: 39531012 Free PMC article. - Detection of Double-Stranded RNA Intermediates During SARS-CoV-2 Infections of Syrian Golden Hamsters with Monoclonal Antibodies and Its Implications for Histopathological Evaluation of In Vivo Studies.
Beythien G, de le Roi M, Stanelle-Bertram S, Armando F, Heydemann L, Rosiak M, Becker S, Lamers MM, Kaiser FK, Haagmans BL, Ciurkiewicz M, Gabriel G, Osterhaus ADME, Baumgärtner W. Beythien G, et al. Int J Mol Sci. 2024 Oct 24;25(21):11425. doi: 10.3390/ijms252111425. Int J Mol Sci. 2024. PMID: 39518980 Free PMC article. - Dicer-2 mutations in Aedes aegypti cells lead to a diminished antiviral function against Rift Valley fever virus and Bunyamwera virus infection.
Dornbusch S, Reuter M, Parry RH, Stern M, Becker SC, Schnettler E. Dornbusch S, et al. J Gen Virol. 2024 Nov;105(11):002046. doi: 10.1099/jgv.0.002046. J Gen Virol. 2024. PMID: 39508739 Free PMC article. - Dothistroma septosporum and Dothistroma pini, the causal agents of Dothistroma needle blight, are infected by multiple viruses.
Trifković M, Hejna O, Kuznetsova A, Mullett M, Jankovský L, Botella L. Trifković M, et al. Virus Res. 2024 Oct 5;350:199476. doi: 10.1016/j.virusres.2024.199476. Online ahead of print. Virus Res. 2024. PMID: 39353468 Free PMC article. - Zika virus NS3 drives the assembly of a viroplasm-like structure.
Sultana T, Zheng C, Morton G, Megraw TL. Sultana T, et al. bioRxiv [Preprint]. 2024 Sep 16:2024.09.16.613201. doi: 10.1101/2024.09.16.613201. bioRxiv. 2024. PMID: 39345390 Free PMC article. Preprint.
References
- Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. - PMC - PubMed
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources