Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment - PubMed (original) (raw)
Comparative Study
. 2006 May;78(5):804-814.
doi: 10.1086/503820. Epub 2006 Mar 20.
Silvia Buervenich 2, Dennis Charney 3, Robert Lipsky 4, A John Rush 5, Alexander F Wilson 6, Alexa J M Sorant 6, George J Papanicolaou 6, Gonzalo Laje 2, Maurizio Fava 7, Madhukar H Trivedi 5, Stephen R Wisniewski 8, Husseini Manji 9
Affiliations
- PMID: 16642436
- PMCID: PMC1474035
- DOI: 10.1086/503820
Comparative Study
Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment
Francis J McMahon et al. Am J Hum Genet. 2006 May.
Abstract
Depressive disorders account for a large and increasing global burden of disease. Although the condition of many patients improves with medication, only a minority experience full remission, and patients whose condition responds to one medication may not have a response to others. Individual variation in antidepressant treatment outcome is, at present, unpredictable but may have a partial genetic basis. We searched for genetic predictors of treatment outcome in 1,953 patients with major depressive disorder who were treated with the antidepressant citalopram in the Sequenced Treatment Alternatives for Depression (STAR*D) study and were prospectively assessed. In a split-sample design, a selection of 68 candidate genes was genotyped, with 768 single-nucleotide-polymorphism markers chosen to detect common genetic variation. We detected significant and reproducible association between treatment outcome and a marker in HTR2A (P range 1 x 10(-6) to 3.7 x 10(-5) in the total sample). Other markers in HTR2A also showed evidence of association with treatment outcome in the total sample. HTR2A encodes the serotonin 2A receptor, which is downregulated by citalopram. Participants who were homozygous for the A allele had an 18% reduction in absolute risk of having no response to treatment, compared with those homozygous for the other allele. The A allele was over six times more frequent in white than in black participants, and treatment was less effective among black participants. The A allele may contribute to racial differences in outcomes of antidepressant treatment. Taken together with prior neurobiological findings, these new genetic data make a compelling case for a key role of HTR2A in the mechanism of antidepressant action.
Figures
Figure 1
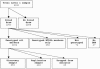
CONSORT chart of genotyping and analysis of STAR*D sample. Samples dropped from analysis comprised 34 subjects who were noncompliant with medications, 19 with initial QIDS-C16 <10 or missing, 5 with missing clinical data, 4 whose molecular and recorded sex did not match, and 3 duplicate samples.
Figure 2
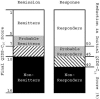
Treatment-outcome phenotypes. Subjects who completed at least 6 wk of treatment with citalopram were assigned a remission and response phenotype that was based on the QIDS-C16 score at the last treatment visit. Those who met score criteria for remission or response after 3–6 wk of treatment were grouped with probable remitters or probable responders, respectively.
Figure 3
Allelic association between treatment outcome and each of 768 SNPs representing 68 candidate genes. Allelic association P values were ranked in the discovery and replication samples, then the ranks were summed across samples. For visual clarity, the inverse of the sum of ranks is shown on the _Y_-axis. The remission phenotype is indicated by the unblackened circles, the response phenotype by the blackened circles. Only rs7997012 met the a priori P value thresholds in both samples. Another SNP, rs2178865, ranked highly in both samples but fell short of the a priori significance level in the replication sample.
Figure 4
Physical positions and LD relationships among SNP markers genotyped in the STAR*D sample. This figure shows the Refseq gene model for HTR2A taken from the UCSC Genome Browser (build 43), juxtaposed on a graphical representation of intermarker
_r_2
values produced by Haploview 3.2.
Similar articles
- Serotonin receptor 2A gene polymorphism (-1438A/G) and short-term treatment response to citalopram.
Choi MJ, Kang RH, Ham BJ, Jeong HY, Lee MS. Choi MJ, et al. Neuropsychobiology. 2005;52(3):155-62. doi: 10.1159/000087847. Epub 2005 Aug 25. Neuropsychobiology. 2005. PMID: 16127283 - Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort.
Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ. Paddock S, et al. Am J Psychiatry. 2007 Aug;164(8):1181-8. doi: 10.1176/appi.ajp.2007.06111790. Am J Psychiatry. 2007. PMID: 17671280 Clinical Trial. - Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment.
Horstmann S, Lucae S, Menke A, Hennings JM, Ising M, Roeske D, Müller-Myhsok B, Holsboer F, Binder EB. Horstmann S, et al. Neuropsychopharmacology. 2010 Feb;35(3):727-40. doi: 10.1038/npp.2009.180. Epub 2009 Nov 18. Neuropsychopharmacology. 2010. PMID: 19924111 Free PMC article. - Genome-wide association studies of antidepressant outcome: a brief review.
Laje G, McMahon FJ. Laje G, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Aug 15;35(7):1553-7. doi: 10.1016/j.pnpbp.2010.11.031. Epub 2010 Nov 27. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21115088 Free PMC article. Review. - Pharmacogenetics of antidepressant response: an update.
Drago A, De Ronchi D, Serretti A. Drago A, et al. Hum Genomics. 2009 Apr;3(3):257-74. doi: 10.1186/1479-7364-3-3-257. Hum Genomics. 2009. PMID: 19403460 Free PMC article. Review.
Cited by
- Serotonin transporter 5-HTTLPR polymorphism and escitalopram treatment response in patients with major depressive disorder.
Jarčušková D, Tkáč I, Hlaváčová N, Yaluri AS, Kozárová M, Habalová V, Klimčáková L, Židzik J, Javorský M, Bednářová A. Jarčušková D, et al. BMC Psychiatry. 2024 Oct 15;24(1):690. doi: 10.1186/s12888-024-06162-8. BMC Psychiatry. 2024. PMID: 39407134 Free PMC article. - A Cohort Study to Evaluate Genetic Predictors of Aromatase Inhibitor Musculoskeletal Symptoms: Results from ECOG-ACRIN E1Z11.
Stearns V, Jegede OA, Chang VT, Skaar TC, Berenberg JL, Nand R, Shafqat A, Jacobs NL, Luginbuhl W, Gilman P, Benson AB 3rd, Goodman JR, Buchschacher GL Jr, Henry NL, Loprinzi CL, Flynn PJ, Mitchell EP, Fisch MJ, Sparano JA, Wagner LI. Stearns V, et al. Clin Cancer Res. 2024 Jul 1;30(13):2709-2718. doi: 10.1158/1078-0432.CCR-23-2137. Clin Cancer Res. 2024. PMID: 38640040 - Relative synonymous codon usage and codon pair analysis of depression associated genes.
Khandia R, Gurjar P, Kamal MA, Greig NH. Khandia R, et al. Sci Rep. 2024 Feb 12;14(1):3502. doi: 10.1038/s41598-024-51909-8. Sci Rep. 2024. PMID: 38346990 Free PMC article. - DRD2, DRD3, and HTR2A Single-Nucleotide Polymorphisms Involvement in High Treatment Resistance to Atypical Antipsychotic Drugs.
Del Casale A, Simmaco M, Modesti MN, Zocchi C, Arena JF, Bilotta I, Alcibiade A, Sarli G, Cutillo L, Antonelli G, La Spina E, De Luca O, Preissner R, Borro M, Gentile G, Girardi P, Pompili M. Del Casale A, et al. Biomedicines. 2023 Jul 24;11(7):2088. doi: 10.3390/biomedicines11072088. Biomedicines. 2023. PMID: 37509727 Free PMC article. - Precision Medicine in Antidepressants Treatment.
Tsermpini EE, Serretti A, Dolžan V. Tsermpini EE, et al. Handb Exp Pharmacol. 2023;280:131-186. doi: 10.1007/164_2023_654. Handb Exp Pharmacol. 2023. PMID: 37195310 Review.
References
Web Resources
- Gene×Environment, Gene×Gene Interaction home page, http://hydra.usc.edu/gxe/ (for Quanto, v. 1.0 beta)
- International HapMap Project, http://www.hapmap.org/
- UCSC Genome Browser, http://genome.ucsc.edu/
References
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, VanMeter S, Harriett AE, Wang Y (2005) Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry 66:974–981 - PubMed
- Marangell LB (2001) Switching antidepressants for treatment-resistant major depression. J Clin Psychiatry Suppl 18 62:12–17 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases