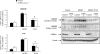Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders - PubMed (original) (raw)
Review
Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders
Benoit Viollet et al. J Physiol. 2006.
Abstract
It is now becoming evident that the liver has an important role in the control of whole body metabolism of energy nutrients. In this review, we focus on recent findings showing that AMP-activated protein kinase (AMPK) plays a major role in the control of hepatic metabolism. AMPK integrates nutritional and hormonal signals to promote energy balance by switching on catabolic pathways and switching off ATP-consuming pathways, both by short-term effects on phosphorylation of regulatory proteins and by long-term effects on gene expression. Activation of AMPK in the liver leads to the stimulation of fatty acid oxidation and inhibition of lipogenesis, glucose production and protein synthesis. Medical interest in the AMPK system has recently increased with the demonstration that AMPK could mediate some of the effects of the fat cell-derived adiponectin and the antidiabetic drugs metformin and thiazolidinediones. These findings reinforce the idea that pharmacological activation of AMPK may provide, through signalling and metabolic and gene expression effects, a new strategy for the management of metabolic hepatic disorders linked to type 2 diabetes and obesity.
Figures
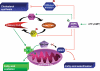
Figure 1. Regulation of lipid metabolism by hepatic AMPK
Activation of AMPK leads to the inhibition of cholesterol synthesis by phosphorylation of HMG-CoA reductase. By inhibiting ACC and activating MCD, AMPK increases fatty acid oxidation via the regulation of levels of malonyl-CoA, which is both a critical precursor for biosynthesis of fatty acids and a potent inhibitor of CPT-1, the enzyme that controls the transfer of long-chain fatty acyl-CoA into the mitochondria. AMPK inhibits also GPAT, the first committed enzyme in glycerolipid synthesis. The net resulting effect of AMPK activation is to inhibit energy-consuming lipogenic pathways (fatty acid, triglyceride and sterol synthesis) in favour of fatty acid oxidation. FA-CoA: fatty acyl-CoA.
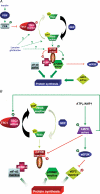
Figure 2. Diagrammatic representation of signalling involved in the regulation of the mTOR/p70S6K pathway by AMPK
A, both insulin and amino acids stimulate the mTOR/p70S6K pathway to promote protein synthesis. Insulin, by activating the phosphatidylinositol-3-kinase/protein kinase B pathway, inhibits TSC2, and so, via the activation of Rheb, induces mTOR activation. On the other hand, the pathway used by amino acids like leucine or glutamine to activate the mTOR pathway is still not well defined. Once activated, mTOR, with the participation of Raptor, is able to phosphorylate 4EBP-1 and p70S6K. B, activated AMPK is able to phosphorylate and activate both TSC2 and eEF2K. Moreover, AMPK can inactivate directly mTOR. GEF: guanylate exchange factor.
Figure 3. Effect of dietary PUFAs on L-PK and ChREBP gene expression and ChREBP localization in livers of AMPKα1α2LS−/− mice
A, quantitative RT-PCR analysis of L-PK and ChREBP gene expression from livers of 24 h-fasted mice (F) and mice re-fed for 18 h with a high carbohydrate diet (HCHO) supplemented or not with PUFAs (PUFA) performed in control (filled bars) and AMPKα1α2LS−/− (open bars) mice. Results are means ±
s.e.m.
; n = 3/group. *Significantly different from mice re-fed with HCHO diet for 18 h (P < 0.005). B, cytosolic and nuclear ChREBP content from livers of 24 h-fasted control and AMPKα1α2LS−/− mice re-fed for 18 h upon HCHO diet supplemented or not with PUFAs. Expression of AMPKα catalytic subunits has been measured by using anti-pan-AMPKα antibodies. β-Actin protein levels are presented as loading control. A representative Western blot is shown; n = 3/group.
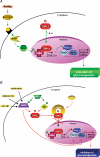
Figure 4. Transcriptional control of gluconeogenesis by TORC2 and AMPK
A, in response to fasting, the cAMP-responsive CREB coactivator TORC2 controls the gluconeogenic programme in liver via its nuclear translocation and association with CREB transcription factor, driving the expression of the PGC1α coactivator. Expression of the coactivator PGC-1α in turn drives the transcription of key gluconeogenic enzymes such as PEPCK and G6Pase in association with the transcription factor HNF4α and the forkhead family activator FoxO1. B, activity of TORC2 is controlled by AMPK and AMPK-related kinase SIK phosphorylation, which determines whether TORC2 becomes localized in the nucleus. Phosphorylated TORC2 is sequestered in the cytoplasm via a phosphorylation-dependent interaction with 14-3-3 proteins. Moreover, AMPK can also control gluconeogenic gene transcription by regulating stability or degradation of HNF4α and FoxO1 transcription factors.
Similar articles
- [Regulation of energy metabolism by AMPK: a novel therapeutic approach for the treatment of metabolic and cardiovascular diseases].
Foretz M, Taleux N, Guigas B, Horman S, Beauloye C, Andreelli F, Bertrand L, Viollet B. Foretz M, et al. Med Sci (Paris). 2006 Apr;22(4):381-8. doi: 10.1051/medsci/2006224381. Med Sci (Paris). 2006. PMID: 16597407 Review. French. - Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders.
Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Viollet B, et al. Diabetes Metab. 2007 Dec;33(6):395-402. doi: 10.1016/j.diabet.2007.10.004. Epub 2007 Nov 7. Diabetes Metab. 2007. PMID: 17997341 Review. - AMP-activated protein kinase in metabolic control and insulin signaling.
Towler MC, Hardie DG. Towler MC, et al. Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review. - AMP-activated protein kinase and type 2 diabetes.
Musi N. Musi N. Curr Med Chem. 2006;13(5):583-9. doi: 10.2174/092986706776055724. Curr Med Chem. 2006. PMID: 16515522 Review. - Role of AMP-activated protein kinase in mechanism of metformin action.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Zhou G, et al. J Clin Invest. 2001 Oct;108(8):1167-74. doi: 10.1172/JCI13505. J Clin Invest. 2001. PMID: 11602624 Free PMC article.
Cited by
- Epigenetics of Hepatic Insulin Resistance.
Maude H, Sanchez-Cabanillas C, Cebola I. Maude H, et al. Front Endocrinol (Lausanne). 2021 May 11;12:681356. doi: 10.3389/fendo.2021.681356. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34046015 Free PMC article. Review. - SCFJFK is functionally linked to obesity and metabolic syndrome.
He L, Yan R, Yang Z, Zhang Y, Liu X, Yang J, Liu X, Liu X, Xia L, Wang Y, Wu J, Wu X, Shan L, Yang X, Liang J, Shang Y, Sun L. He L, et al. EMBO Rep. 2021 Jul 5;22(7):e52036. doi: 10.15252/embr.202052036. Epub 2021 Jun 11. EMBO Rep. 2021. PMID: 34114325 Free PMC article. - Metformin enhances LDL-cholesterol uptake by suppressing the expression of the pro-protein convertase subtilisin/kexin type 9 (PCSK9) in liver cells.
Ali A, Unnikannan H, Shafarin J, Bajbouj K, Taneera J, Muhammad JS, Hasan H, Salehi A, Awadallah S, Hamad M. Ali A, et al. Endocrine. 2022 Jun;76(3):543-557. doi: 10.1007/s12020-022-03022-x. Epub 2022 Mar 2. Endocrine. 2022. PMID: 35237909 - The contrary intracellular and extracellular functions of PEDF in HCC development.
Li C, Huang Z, Zhu L, Yu X, Gao T, Feng J, Hong H, Yin H, Zhou T, Qi W, Yang Z, Liu C, Yang X, Gao G. Li C, et al. Cell Death Dis. 2019 Oct 3;10(10):742. doi: 10.1038/s41419-019-1976-4. Cell Death Dis. 2019. PMID: 31582735 Free PMC article. - Exendin-4 Improves Diabetic Kidney Disease in C57BL/6 Mice Independent of Brown Adipose Tissue Activation.
Fang S, Cai Y, Lyu F, Zhang H, Wu C, Zeng Y, Fan C, Zou S, Zhang Y, Li P, Wang L, YaomingXue, Guan M. Fang S, et al. J Diabetes Res. 2020 Feb 3;2020:9084567. doi: 10.1155/2020/9084567. eCollection 2020. J Diabetes Res. 2020. PMID: 32090125 Free PMC article.
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
- Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not by insulin. Endocrinology. 2006;147:2432–2441. - PubMed
- Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–E800. - PubMed
- Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364–1370. - PubMed
- Barthel A, Schmoll D, Kruger KD, Roth RA, Joost HG. Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology. 2002;143:3183–3186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical