The global distribution of yellow fever and dengue - PubMed (original) (raw)
Review
The global distribution of yellow fever and dengue
D J Rogers et al. Adv Parasitol. 2006.
Abstract
Yellow fever has been subjected to partial control for decades, but there are signs that case numbers are now increasing globally, with the risk of local epidemic outbreaks. Dengue case numbers have also increased dramatically during the past 40 years and different serotypes have invaded new geographical areas. Despite the temporal changes in these closely related diseases, and their enormous public health impact, few attempts have been made to collect a comprehensive dataset of their spatial and temporal distributions. For this review, records of the occurrence of both diseases during the 20th century have been collected together and are used to define their climatic limits using remotely sensed satellite data within a discriminant analytical model framework. The resulting risk maps for these two diseases identify their different environmental requirements, and throw some light on their potential for co-occurrence in Africa and South East Asia.
Figures
Figure 1
Yellow fever transmission cycles for (a) South America and (b) Africa. Simian host species in South America include Alouatta sp., Ateles sp., Callithrix sp., Cebus sp. and Saimiri sp. while those in Africa include Colobus abyssinicus, C. polycomos, C. badius, Cercopithicus sp., Cercocebus sp., Erythrocebus sp., Papio papio, P. anubis and Pan troglodytes.
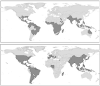
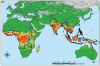
Figure 2
Global distribution of Aedes aegypti (top) and Aedes albopictus (bottom), two important vector species of yellow fever and dengue. Aedes albopictus distributions are provided at national scale. Australia, New Zealand and South Africa have all reported mosquito interception at ports (see Tatem et al., this volume, pp. 293–343). Source: Center for International Earth Science Information Network (CIESIN) [
http://www.ciesin.org/docs/001-613/map15.gif
], supplemented with information from Gratz (2004); Gubler (2003); Lounibos (2002); Medlock et al. (2005); Moore (1999); and Moore and Mitchell (1997).
Figure 3
Plot of the number of yellow fever cases and the number of vaccinations in French West Africa for the period of 1934–1953, demonstrating the effect of the French vaccine on case numbers. Source: Vainio and Cutts (1998).
Figure 4
Yellow fever vaccination certificate requirements by country. □E1 requirements,  E2 requirements
E2 requirements  E3 requirements
E3 requirements  E4 requirements ■E5 requirements. There are five levels of certification: E1—immunisation is an essential requirement for entry to the country concerned and a certificate is required, except for infants under one year, E2—immunisation is an essential requirement for entry to the country concerned and a certificate is required (except for infants under one year) unless arriving from non-infected areas and staying for less than two weeks, E3—immunisation is an essential requirement for entry to the country concerned and a certificate is required if the traveller arrives from an infected country or area, E4—immunisation is an essential requirement for entry to the country concerned and a certificate is required if arriving within six days of having visited an infected country, E5—immunisation is an essential requirement for entry to the country concerned and a certificate is required for entry to the country from endemic areas, travelling to Easter island. Source: From data obtained at [
E4 requirements ■E5 requirements. There are five levels of certification: E1—immunisation is an essential requirement for entry to the country concerned and a certificate is required, except for infants under one year, E2—immunisation is an essential requirement for entry to the country concerned and a certificate is required (except for infants under one year) unless arriving from non-infected areas and staying for less than two weeks, E3—immunisation is an essential requirement for entry to the country concerned and a certificate is required if the traveller arrives from an infected country or area, E4—immunisation is an essential requirement for entry to the country concerned and a certificate is required if arriving within six days of having visited an infected country, E5—immunisation is an essential requirement for entry to the country concerned and a certificate is required for entry to the country from endemic areas, travelling to Easter island. Source: From data obtained at [
].
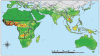
Figure 5
Yellow fever outbreak distribution for 1900–1959 (top) and 1960–2005 (bottom). The maps are displayed between 40°N and 40°S as these latitudes encompass all known areas of the disease.
Figure 6
All-sera dengue outbreak distribution for 1960–2005. The map is displayed between 40°N and 40°S as these latitudes encompass all known areas of the disease.
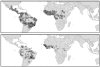
Plate 6.7
Risk map for yellow fever. This risk map is the average of 100 bootstrap models each based on a sample of 300 presence and 300 absence pixels selected at random with replacement from the training set for this disease. Risk is on a probability scale from zero to 1.0. Probabilities from 0.0 to 0.49 are coloured green (darker to lighter) and indicate conditions not suitable for the disease (i.e. predicted absence of disease). Probabilities from 0.50 to 1.0 are coloured yellow through to dark red, indicating conditions increasingly suitable for the disease. The database observations of presence are indicated by the blue dots and the WHO 2003 map for yellow fever by the thick black outline.
Plate 6.7
Risk map for yellow fever. This risk map is the average of 100 bootstrap models each based on a sample of 300 presence and 300 absence pixels selected at random with replacement from the training set for this disease. Risk is on a probability scale from zero to 1.0. Probabilities from 0.0 to 0.49 are coloured green (darker to lighter) and indicate conditions not suitable for the disease (i.e. predicted absence of disease). Probabilities from 0.50 to 1.0 are coloured yellow through to dark red, indicating conditions increasingly suitable for the disease. The database observations of presence are indicated by the blue dots and the WHO 2003 map for yellow fever by the thick black outline.
Plate 6.8
Risk map for dengue. This risk map is the average of 100 bootstrap models each based on a sample of 900 presence and 900 absence pixels selected at random with replacement from the training set for this disease. Risk is on a probability scale from zero to 1.0. Probabilities from 0.0 to 0.49 are coloured green (darker to lighter) and indicate conditions not suitable for the disease (i.e. predicted absence of disease). Probabilities from 0.50 to 1.0 are coloured yellow through to dark red, indicating conditions increasingly suitable for the disease. The database observations of presence are indicated by the blue dots and the WHO 2003 map for dengue by the thick black outline.
Plate 6.8
Risk map for dengue. This risk map is the average of 100 bootstrap models each based on a sample of 900 presence and 900 absence pixels selected at random with replacement from the training set for this disease. Risk is on a probability scale from zero to 1.0. Probabilities from 0.0 to 0.49 are coloured green (darker to lighter) and indicate conditions not suitable for the disease (i.e. predicted absence of disease). Probabilities from 0.50 to 1.0 are coloured yellow through to dark red, indicating conditions increasingly suitable for the disease. The database observations of presence are indicated by the blue dots and the WHO 2003 map for dengue by the thick black outline.
Plate 1.3
Results from the 100 bootstrap models for (left) Rift Valley Fever, (middle) Yellow Fever and (right) Dengue. Each row in the image refers to one of the models, which are arranged in rank order, with 1 (lowest AICc value) at the top and 100 (highest AICc value) at the bottom. Each of the 31 columns on the right of the image indicates one of the satellite predictor variables available to describe the disease. The first column of these 31 columns is for the digital elevation layer or DEM. There then follow three sets of 10 columns referring to the Fourier-processed AVHRR MIR, LST and NDVI imagery. These layers are in the following order: mean, phase of annual cycle, amplitude of annual cycle; phase of bi-annual cycle, amplitude of bi-annual cycle; phase of tri-annual cycle, amplitude of tri-annual cycle; maximum of fitted Fourier cycles (summed annual to tri-annual), minimum of fitted Fourier cycles and variance of the original signal. In any single model (row) the top (i.e. first selected) predictor variable is coloured red, the second most important variable is coloured orange and so on according to the rainbow colour scale to the right of the image. Variables not chosen in any model are not coloured at all in that row. The red line down the first image indicates variable 14 in the variable list, which is the annual amplitude of LST. This variable is consistently chosen in all RVF models, and is usually the most important variable, but there is no other single variable which is consistently chosen second. (The left-most column refers to the model number in the sequence; this, and the grey area to the left of the variable columns should be ignored.) The other two images may be similarly interpreted (see Rogers et al., this volume, pp. 181–220, for more details).
Figure 10
(a) Total numbers of humans within each category of yellow fever or dengue risk as shown in Figures 8 and 9 (thick lines, millions scale) and numbers of 0.10° grid squares within each risk category (dashed lines, thousands scale). (b) Mean human population density per 0.10° grid square for each category of yellow fever and dengue risk.
Figure 11
Reported number of yellow fever outbreaks per year per continent. Source: Weekly Epidemiological Record archives.
Similar articles
- The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle?
Gubler DJ. Gubler DJ. Comp Immunol Microbiol Infect Dis. 2004 Sep;27(5):319-30. doi: 10.1016/j.cimid.2004.03.013. Comp Immunol Microbiol Infect Dis. 2004. PMID: 15225982 Review. - Why dengue and yellow fever coexist in some areas of the world and not in others?
Amaku M, Coutinho FA, Massad E. Amaku M, et al. Biosystems. 2011 Nov;106(2-3):111-20. doi: 10.1016/j.biosystems.2011.07.004. Epub 2011 Aug 2. Biosystems. 2011. PMID: 21839800 - Aedes (Stegomyia) aegypti in the continental United States: a vector at the cool margin of its geographic range.
Eisen L, Moore CG. Eisen L, et al. J Med Entomol. 2013 May;50(3):467-78. doi: 10.1603/me12245. J Med Entomol. 2013. PMID: 23802440 - Using global maps to predict the risk of dengue in Europe.
Rogers DJ, Suk JE, Semenza JC. Rogers DJ, et al. Acta Trop. 2014 Jan;129:1-14. doi: 10.1016/j.actatropica.2013.08.008. Epub 2013 Aug 21. Acta Trop. 2014. PMID: 23973561 - Is there a risk of yellow fever virus transmission in South Asian countries with hyperendemic dengue?
Agampodi SB, Wickramage K. Agampodi SB, et al. Biomed Res Int. 2013;2013:905043. doi: 10.1155/2013/905043. Epub 2013 Dec 3. Biomed Res Int. 2013. PMID: 24367789 Free PMC article. Review.
Cited by
- A Systematic Review on Modeling Methods and Influential Factors for Mapping Dengue-Related Risk in Urban Settings.
Yin S, Ren C, Shi Y, Hua J, Yuan HY, Tian LW. Yin S, et al. Int J Environ Res Public Health. 2022 Nov 18;19(22):15265. doi: 10.3390/ijerph192215265. Int J Environ Res Public Health. 2022. PMID: 36429980 Free PMC article. Review. - Vaccination and Therapeutics: Responding to the Changing Epidemiology of Yellow Fever.
Bifani AM, Ong EZ, de Alwis R. Bifani AM, et al. Curr Treat Options Infect Dis. 2020;12(3):349-360. doi: 10.1007/s40506-020-00232-7. Epub 2020 Jul 10. Curr Treat Options Infect Dis. 2020. PMID: 32837338 Free PMC article. Review. - The Neglect and Fast Spread of Some Arboviruses: A Note for Healthcare Providers in Nigeria.
Kolawole OM, Seriki AA, Irekeola AA, Ogah JI. Kolawole OM, et al. Diseases. 2018 Nov 5;6(4):99. doi: 10.3390/diseases6040099. Diseases. 2018. PMID: 30400643 Free PMC article. Review. - A community-level investigation following a yellow fever virus outbreak in South Omo Zone, South-West Ethiopia.
Mulchandani R, Massebo F, Bocho F, Jeffries CL, Walker T, Messenger LA. Mulchandani R, et al. PeerJ. 2019 Feb 20;7:e6466. doi: 10.7717/peerj.6466. eCollection 2019. PeerJ. 2019. PMID: 30809451 Free PMC article. - A systematic review of the data, methods and environmental covariates used to map Aedes-borne arbovirus transmission risk.
Lim AY, Jafari Y, Caldwell JM, Clapham HE, Gaythorpe KAM, Hussain-Alkhateeb L, Johansson MA, Kraemer MUG, Maude RJ, McCormack CP, Messina JP, Mordecai EA, Rabe IB, Reiner RC Jr, Ryan SJ, Salje H, Semenza JC, Rojas DP, Brady OJ. Lim AY, et al. BMC Infect Dis. 2023 Oct 20;23(1):708. doi: 10.1186/s12879-023-08717-8. BMC Infect Dis. 2023. PMID: 37864153 Free PMC article.
References
- Aitken TH, Tesh RB, Beaty BJ, Rosen L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti) American Journal of Tropical Medicine and Hygiene. 1979;28:119–121. - PubMed
- Armstrong C. Dengue fever. Public Health Reports. 1923;38:1750–1784.
- Balfour A. Tropical problems in the New World. Transactions of the Royal Society for Tropical Medicine and Hygiene. 1915;8:75.
- Barros ML, Boecken G. Jungle yellow fever in the central Amazon. Lancet. 1996;348:969–970. - PubMed
- Beaty BJ, Aitken THG. In vitro transmission of yellow fever virus by geographic strains of Aedes aegypti. Mosquito News. 1979;39:232–238.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous