In vivo, competitive blockade of N-methyl-D-aspartate receptors induces rapid changes in filamentous actin and drebrin A distributions within dendritic spines of adult rat cortex - PubMed (original) (raw)
Comparative Study
In vivo, competitive blockade of N-methyl-D-aspartate receptors induces rapid changes in filamentous actin and drebrin A distributions within dendritic spines of adult rat cortex
S Fujisawa et al. Neuroscience. 2006.
Abstract
In vitro studies have demonstrated that prolonged N-methyl-D-aspartate receptor (NMDAR) blockade triggers a homeostatic up-regulation of NMDARs at synapses. Such upregulation can also be seen within 30 min in vivo in adult rats, implicating trafficking of reserve pools of NMDARs. Here, we evaluated the involvement of filamentous actin (F-actin), the major cytoskeletal component in spines, in this rapid in vivo homeostatic response, using biotinylated phalloidin as its probe. We also immuno-labeled spines for drebrin A, an F-actin-binding protein found at excitatory synapses and with a proposed role of modulating F-actin's cross-linking with one another and interactions with NMDARs. Quantitative 2-D analysis of ultrastructural images revealed that NMDAR blockade increased filamentous actin labeling per spine by 62.5% (P<0.005). The proportion of dendritic spines immuno-labeled for drebrin A also increased significantly, from 67.5% to 85% following NMDAR blockade (P<0.001), especially among larger spines. The frequency distributions of spine widths and postsynaptic density lengths were not affected by the D-(+)-2-amino-5-phosphonopentanoic acid (D-APV) treatment. However, the average postsynaptic density length was reduced by 25 nm among the fewer, drebrin A immuno-negative spines, indicating that drebrin A confers stability to synapse size. We propose that, in a homeostatic in vivo response, increases of drebrin A and F-actin within spines can enhance NMDAR trafficking by reducing cytoskeletal rigidity within the spine cytoplasm without changing the overt morphology of axo-spinous synapses. Alternatively or in addition, the cytoskeletal redistribution within spine cytoplasm may be triggered by the D-APV-induced, homeostatic up-regulation of NMDAR.
Figures
Fig. 1
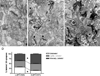
Electron micrographs show phalloidin-labeling of F-actin in presynaptic axon terminals and postsynaptic spines of
l
-APV- and
d
-APV-treated tissues. The micrographs in these figures were derived from tissues of a paraformaldehyde-fixed animal, sampled strictly along the tissue-Epon interface (asterisks on the Epon-side). Arrowheads, indicating synapses, reside in the presynaptic terminals and point to PSDs. White arrowheads point to unlabeled synapses. Black arrowheads point to labeled synapses; i.e. those with one or more SIG particles in pre- and/or postsynaptic terminals. Post, postsynaptic spine; Pre, presynaptic axon terminal. Scale bar=200 nm. (A) Cytochemical control tissue from
d
-APV-treated hemisphere, incubated in a buffer lacking biotinylated phalloidin but containing gold-conjugated anti-biotin antibody. There was very little SIG labeling; none of them were found at synapses. (B) Pharmacological control,
l
-APV-treated tissue. Phalloidin labels were found in dendritic shafts, spines and axon terminals. Those found in dendritic spines were tallied as “postsynaptic labels,” while those of the axon terminals were tallied as “presynaptic labels.” In this example, two out of three synapses within this field show no detectable phalloidin-binding sites. (C)
d
-APV-treated tissue. More synapses were labeled with SIG particles and each labeled synapse contained more particles, compared with the
l
-APV-treated tissue.
Fig. 2
The proportion of phalloidin-labeled synapses increases after
d
-APV application. For every 20 synapses encountered, the percentage of immuno-labeled synapses was calculated. The bar graphs show the mean percentage with S.E.M. as error bars. The numbers in parentheses below the bars correspond to the N for that data set. The distributions were compared between
l
-APV and
d
-APV treatments of single animals. Student’s _t_-test showed significant increase in the proportion of labeled synapses, in both glutaraldehyde- and paraformaldehyde-fixed tissues. * Indicates P<0.001.
Fig. 3
The number of SIG particles labeling F-actin in the synaptic subcellular domains is greater in the
d
-APV-treated tissues. Top two graphs (A and B) represent the average number of SIG particles found within presynaptic profile, postsynaptic profile and across both micro-domains of each synapse (“All”). Data in graph A were taken from tissues of animals perfused with 4% paraformaldehyde–1% glutaraldehyde mixture (_n_=3). Data in graph B were from paraformaldehyde-fixed animals (_n_=4). Error bars show the standard error of means across synapses. Lighter-colored bars are data from
l
-APV-treated tissues from each animal, and darker-shaded bars are those from
d
-APV-treated tissues. The total number of synapses examined for both tissues is shown in parentheses next to the legend. Statistical analysis comparing
l
- and
d
-APV-treated tissues was performed for each micro-domain of each animal separately, using the non-parametric Mann-Whitney U test. Single asterisk indicates P<0.001. Panel C combines the data from both groups of data, by averaging the percent change from
l
-APV from all of the glutaraldehyde- and paraformaldehyde-fixed animals examined (_n_=7). Single-sample _t_-test was used to determine whether the changes were significantly different from zero; single star denotes P<0.005.
Fig. 4
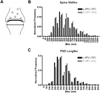
d
-APV treatment induces significant increases in drebrin A-labeled spines. All micrographs were taken from a representative animal. As with phalloidin-labeled tissues, pictures were taken along the tissue–Epon interface. All arrows indicating synapses are in presynaptic terminals, pointing at PSDs. White arrowheads point to unlabeled synapses; black arrows to lightly-labeled synapses; and black arrowheads to intensely-labeled synapses. Drebrin A labeling, when present, was always found within the postsynaptic profile and was never found presynaptically. (A) Control tissues incubated in a buffer without the primary antibody but otherwise treated identically to experimental tissue. No synapse was labeled by DAB (white arrows). (B)
l
-APV-treated tissue. Most synapses were labeled with DAB, but many of them were only lightly labeled (black arrows). (C)
d
-APV-treated tissue. There were more intensely labeled synapses (black arrowheads). (D) Each bar represents the proportion of unlabeled (white), lightly-labeled (gray) and intensely-labeled (dark gray) spines among all spines encountered (indicated in parentheses on the x axis). The proportion of unlabeled spines was significantly reduced within
d
-APV tissues, while the proportion of intensely-labeled synapses was significantly increased. Asterisk indicates P<0.001 by Student’s _t_-test.
Fig. 5
d
-APV treatment did not cause enlargement of spine heads or lengthening of PSDs. (A) Schematic drawing of how spine widths were measured on electron micrographs taken from glutaraldehyde- and osmium-fixed, drebrin immuno-labeled tissue. All spines encountered, whether or not drebrin A-labeled, were included in the sample. A line parallel to the PSD and postsynaptic membrane was drawn at the widest part of the spine head (arrow). Then length of this line was measured in nanometers. Additionally, the length of the PSD was measured by following any curvatures that were present along the synaptic cleft. (B, C) Normalized frequency distributions of spine widths and PSD lengths, respectively, binned by 50 nm. The numbers in parentheses in the legend represent the N for each group. There were no statistical differences in frequency distributions from the
l
- and
d
-APV tissues.
Similar articles
- Drebrin a knockout eliminates the rapid form of homeostatic synaptic plasticity at excitatory synapses of intact adult cerebral cortex.
Aoki C, Kojima N, Sabaliauskas N, Shah L, Ahmed TH, Oakford J, Ahmed T, Yamazaki H, Hanamura K, Shirao T. Aoki C, et al. J Comp Neurol. 2009 Nov 1;517(1):105-21. doi: 10.1002/cne.22137. J Comp Neurol. 2009. PMID: 19711416 Free PMC article. - Drebrin a content correlates with spine head size in the adult mouse cerebral cortex.
Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T. Kobayashi C, et al. J Comp Neurol. 2007 Aug 10;503(5):618-26. doi: 10.1002/cne.21408. J Comp Neurol. 2007. PMID: 17559090 Free PMC article. - Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.
Aoki C, Sherpa AD. Aoki C, et al. Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review. - Activation of N-methyl-D-aspartate receptor induces a shift of drebrin distribution: disappearance from dendritic spines and appearance in dendritic shafts.
Sekino Y, Tanaka S, Hanamura K, Yamazaki H, Sasagawa Y, Xue Y, Hayashi K, Shirao T. Sekino Y, et al. Mol Cell Neurosci. 2006 Mar;31(3):493-504. doi: 10.1016/j.mcn.2005.11.003. Epub 2005 Dec 20. Mol Cell Neurosci. 2006. PMID: 16368245 - The role of drebrin in dendritic spines.
Koganezawa N, Hanamura K, Sekino Y, Shirao T. Koganezawa N, et al. Mol Cell Neurosci. 2017 Oct;84:85-92. doi: 10.1016/j.mcn.2017.01.004. Epub 2017 Feb 1. Mol Cell Neurosci. 2017. PMID: 28161364 Review.
Cited by
- Dysregulated Signaling at Postsynaptic Density: A Systematic Review and Translational Appraisal for the Pathophysiology, Clinics, and Antipsychotics' Treatment of Schizophrenia.
de Bartolomeis A, Vellucci L, De Simone G, Mazza B, Barone A, Ciccarelli M. de Bartolomeis A, et al. Cells. 2023 Feb 10;12(4):574. doi: 10.3390/cells12040574. Cells. 2023. PMID: 36831241 Free PMC article. Review. - Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier.
Giardino L, Armelloni S, Corbelli A, Mattinzoli D, Zennaro C, Guerrot D, Tourrel F, Ikehata M, Li M, Berra S, Carraro M, Messa P, Rastaldi MP. Giardino L, et al. J Am Soc Nephrol. 2009 Sep;20(9):1929-40. doi: 10.1681/ASN.2008121286. Epub 2009 Jul 2. J Am Soc Nephrol. 2009. PMID: 19578006 Free PMC article. - Drebrin a knockout eliminates the rapid form of homeostatic synaptic plasticity at excitatory synapses of intact adult cerebral cortex.
Aoki C, Kojima N, Sabaliauskas N, Shah L, Ahmed TH, Oakford J, Ahmed T, Yamazaki H, Hanamura K, Shirao T. Aoki C, et al. J Comp Neurol. 2009 Nov 1;517(1):105-21. doi: 10.1002/cne.22137. J Comp Neurol. 2009. PMID: 19711416 Free PMC article. - Actin and Actin-Binding Proteins: Masters of Dendritic Spine Formation, Morphology, and Function.
Lin WH, Webb DJ. Lin WH, et al. Open Neurosci J. 2009 Jan 1;3:54-66. doi: 10.2174/1874082000903020054. Open Neurosci J. 2009. PMID: 20717495 Free PMC article. - Drebrin a content correlates with spine head size in the adult mouse cerebral cortex.
Kobayashi C, Aoki C, Kojima N, Yamazaki H, Shirao T. Kobayashi C, et al. J Comp Neurol. 2007 Aug 10;503(5):618-26. doi: 10.1002/cne.21408. J Comp Neurol. 2007. PMID: 17559090 Free PMC article.
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. - PubMed
- Adam G, Matus A. Role of actin in the organisation of brain postsynaptic densities. Brain Res Mol Brain Res. 1996;43:246–250. - PubMed
- Aoki C, Fujisawa S, Mahadomrongkul V, Shah PJ, Nader K, Erisir A. NMDA receptor blockade in intact adult cortex increases trafficking of NR2A subunits into spines, postsynaptic densities, and axon terminals. Brain Res. 2003;963:139–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-EY13145/EY/NEI NIH HHS/United States
- R01 EY008055-09/EY/NEI NIH HHS/United States
- R01 NS041091/NS/NINDS NIH HHS/United States
- P30 EY013079/EY/NEI NIH HHS/United States
- R01 EY013145/EY/NEI NIH HHS/United States
- R25 NS080686/NS/NINDS NIH HHS/United States
- P30 EY13079/EY/NEI NIH HHS/United States
- R01 NS041091-02/NS/NINDS NIH HHS/United States
- P30 EY013079-069003/EY/NEI NIH HHS/United States
- R01-NS41091/NS/NINDS NIH HHS/United States
- R01 EY013145-02/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources