Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity - PubMed (original) (raw)
. 2006 May 1;66(9):4904-12.
doi: 10.1158/0008-5472.CAN-05-2813.
Adi Diab, Miguel-Angel Perales, Jedd D Wolchok, Gabrielle Rizzuto, Taha Merghoub, Deonka Huggins, Cailian Liu, Mary Jo Turk, Nicholas P Restifo, Shimon Sakaguchi, Alan N Houghton
Affiliations
- PMID: 16651447
- PMCID: PMC2242844
- DOI: 10.1158/0008-5472.CAN-05-2813
Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity
Adam D Cohen et al. Cancer Res. 2006.
Abstract
Immunization of mice with plasmids encoding xenogeneic orthologues of tumor differentiation antigens can break immune ignorance and tolerance to self and induce protective tumor immunity. We sought to improve on this strategy by combining xenogeneic DNA vaccination with an agonist anti-glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) monoclonal antibody (mAb), DTA-1, which has been shown previously both to costimulate activated effector CD4(+) and CD8(+) T cells and to inhibit the suppressive activity of CD4(+)CD25(+) regulatory T cells. We found that ligation of GITR with DTA-1 just before the second, but not the first, of 3 weekly DNA immunizations enhanced primary CD8(+) T-cell responses against the melanoma differentiation antigens gp100 and tyrosinase-related protein 2/dopachrome tautomerase and increased protection from a lethal challenge with B16 melanoma. This improved tumor immunity was associated with a modest increase in focal autoimmunity, manifested as autoimmune hypopigmentation. DTA-1 administration on this schedule also led to prolonged persistence of the antigen-specific CD8(+) T cells as well as to an enhanced recall CD8(+) T-cell response to a booster vaccination given 4 weeks after the primary immunization series. Giving the anti-GITR mAb both during primary immunization and at the time of booster vaccination increased the recall response even further. Finally, this effect on vaccine-induced CD8(+) T-cell responses was partially independent of CD4(+) T cells (both helper and regulatory), consistent with a direct costimulatory effect on the effector CD8(+) cells themselves.
Figures
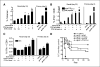
Figure 1
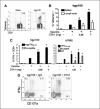
Anti-GITR mAb enhances DNA vaccine-induced CD8+ T-cell responses. C57BL/6 mice (n = 3 per group) were immunized by gene gun thrice weekly with hgp100 or hTRP2-expressing plasmids, with DTA-1 or rat IgG control antibody injected i.p. 1 day before the second immunization. Antigen-specific CD8+ T-cell responses were assessed in spleen and/or draining lymph nodes 5 days after the final immunization. A, representative staining with hgp10025−33-Db-tetramer, showing gated CD8+ lymphocytes. B, splenocytes and lymph node cells from naïve mice or mice immunized with hgp100 DNA + IgG (1 mg) or DTA-1 (0.25 or 1 mg) were stained with hgp100 tetramer and anti-CD8 antibody. Columns, mean percentage of CD8+ lymphocytes that are tetramer+. C, intracellular cytokine assay. Splenocytes from naïve, hgp100-immunized (left), or hTRP2-immunized mice (right) were stimulated overnight with EL4 cells either with or without 1 μg/mL of the indicated peptides or with irradiated B16 melanoma cells (hTRP2 only) then stained for intracellular IFN-γ production. Columns, mean percentage of CD8+ lymphocytes that are IFN-γ+. D, representative staining from CD107a mobilization assay. Splenocytes from hgp100-immunized mice treated with 1 mg control IgG or DTA-1 were cultured for 6 hours with EL4 cells and either with or without 1 μg/mL mouse gp10025−33 peptide and stained for intracellular IFN-γ and CD107a. Gated CD8+ lymphocytes from peptide-stimulated wells. *, P < 0.005, compared with vaccine + IgG control by two-sided Student's t test. Columns, representative of six (hgp100) and three (hTRP2) independent experiments; bars, SE.
Figure 2
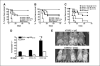
Tumor immunity and autoimmunity following DNA immunization ± anti-GITR mAb. Ten mice per group were not treated or immunized thrice weekly with hgp100 (A and C) or hTRP2 (B) DNA + control IgG or DTA-1. Mice were challenged i.d. with 50,000 B16 melanoma cells 5 days after the final immunization and monitored every 2 to 3 days for tumor growth. Kaplan-Meier curves of tumor-free survival are shown. A and B, control IgG (1 mg) or DTA-1 at the indicated doses was injected 1 day before the second immunization. C, DTA-1 (1 mg) was injected 1 day before the first or second immunization. Control IgG (1 mg) was given before the first immunization. D, AICD assay: naïve Thy1.1+ pmel-1 splenocytes (30 × 106) per mouse were adoptively transferred into naïve Thy1.2+ recipients. DTA-1 or control IgG (1 mg) was injected on day 0 (4 hours after transfer) or 7, and mice were immunized with hgp100 DNA on days 1 and 8. Draining lymph nodes were harvested on day 12, restimulated overnight with EL4 cells either with or without 1 μg/mL mgp10025−33 peptide and stained with anti-CD8, anti-Thy1.1, Annexin V, and DAPI. Columns, percentage from replicate wells of gated DAPI−CD8+Thy1.1+ cells that were Annexin V positive; bars, SE. *, P = 0.02, compared with IgG group; ‡, P = 0.03, compared with DTA-1 day 7 group. E, autoimmune hypopigmentation after hTRP2 immunization. Surviving mice from experiment in (B) were sacrificed on day 72, placed on a black background, and scanned using a flatbed scanner with identical settings for each scanned image and without subsequent manipulation of images other than cropping/size adjustment. Mice immunized with hTRP2 and control IgG or 0.25 mg DTA-1. Dashed lines, border of hypopigmentation for a representative animal from each group. A similar degree of hypopigmentation was seen in mice receiving 1 mg DTA-1 (results not shown). Representative of four experiments for (A) and three experiments for (B) and (C).
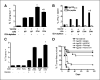
Figure 3
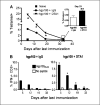
Persistence of gp100-specific CD8+ T cells following hgp100 DNA immunization ± anti-GITR mAb. Splenocytes were harvested from mice (n = 3 per group) 5, 12, 19, or 33 days following completion of 3 weekly immunizations with hgp100 DNA + control IgG or DTA-1 (injected 1 day before the second immunization) or from naïve mice. Immunizations were staggered so that T-cell responses were assessed in all groups on the same day. A, tetramer assay: mean percentage of CD8+ lymphocytes, which are hgp100-tetramer+, is depicted for each time point. Bars, hidden by symbols. Inset, day 33 responses. B, intracellular cytokine assay. Mean percentage of CD8+ lymphocytes, which produce IFN-γ following overnight peptide restimulation, is depicted for each time point. *, P < 0.005; **, P = 0.02, by two-tailed Student's t test, compared with hgp100 + IgG control at corresponding time point. Representative of two independent experiments.
Figure 4
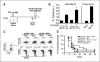
Effect of anti-GITR mAb given during primary hgp100 DNA immunization on memory CD8+ T-cell responses. A, immunization schedule. Mice (n = 3 per group for T-cell assays and n = 10−12 per group for tumor challenge) were immunized thrice weekly with hgp100 DNA + control IgG or DTA-1 (1 mg) injected 1 day before the second immunization then rested for 4 weeks followed by a single hgp100 DNA secondary boost or no boost on day 28 and by B16 tumor challenge or assessment of recall CD8+ T-cell responses 5 days later (day 33). B, tetramer assay. Left, mean percentage of CD8+ lymph node cells that are hgp100-tetramer+ for each group; right, primary response. Percentage of tetramer+CD8+ cells harvested from mice during the peak effector response (5 days following a primary immunization series with hgp100) or from unimmunized mice. Similar pattern of responses was seen in the spleen as well as by intracellular cytokine staining for IFN-γ (data not shown). *, P = 0.004, compared with hgp100 + IgG + day 28 boost group. Columns, mean from triplicate wells; bars, SE. C, phenotype of memory gp100-specific CD8+ T cells. Representative plots, gated tetramer+CD8+ cells stained with anti-CD44, anti-CD62L, and/or CD122 antibodies. Numbers, percentage of tetramer+CD8+ cells residing in particular quadrants. D, tumor challenge. Mice received 50,000 B16 melanoma cells i.d. on day 33 following the primary immunization series (5 days after the boost in boosted groups) and were monitored every 2 to 3 days for tumor growth. Kaplan-Meier curves of tumor-free survival are shown.
Figure 5
Effect of anti-GITR mAb given at time of secondary booster vaccination on memory CD8+ T-cell responses. Mice (n = 3 per group) were immunized with hgp100 DNA as per schedule in Fig. 4_A_, except that some groups were also injected with 1 mg DTA-1 or control IgG 1 day following the day 28 booster vaccination. Recall CD8+ T-cell responses were assessed on day 33. Control groups included naïve mice and mice immunized thrice weekly with hgp100 DNA + 1 mg DTA-1 or IgG and assessed at day 5 after immunization (primary response). A, hgp100-tetramer assay. Columns, mean percentage from triplicate wells of CD8+ splenocytes that are tetramer+; bars, SE. B and C, intracellular cytokine assay. Columns, mean percentage from triplicate wells of CD8+ splenocytes that secreted IFN-γ following restimulation either with or without 1 μg/mL mouse gp10025−33 peptide (B) or irradiated B16 melanoma cells (C); bars, SE. *, P < 0.05, compared with boosted group receiving no DTA-1 (column 3); ‡, P < 0.01, compared with boosted groups receiving DTA-1 either during primary immunization or at boost but not both. D, tumor challenge: mice (n = 15 per group) received 50,000 B16 melanoma cells i.d. on day 33 following the primary immunization series (day 5 after the boost) and were monitored every 2 to 3 days for tumor growth. Kaplan-Meier curves of tumor-free survival are shown. Antibody injected during the primary immunization is listed first followed by the antibody injected after the boost.
Figure 6
Depletion of CD4+ or CD25+ cells before anti-GITR mAb treatment does not abrogate enhancement of CD8+ T-cell responses. A and B, mice (n = 3 per group) were immunized with hgp100 DNA thrice weekly (days 0, 7, and 14) + 1 mg control IgG or DTA-1 injected 1 day before the second immunization (day 6). Some groups received 0.25 mg depleting anti-CD4 mAb (GK1.5) i.p. on days 4 and 11, thereby depleting CD4+ cells before and during GITR ligation by DTA-1. Splenocytes and lymph node cells were harvested 5 days after the final immunization (day 19) and assessed for CD8+ T-cell responses. Columns, mean percentage of triplicate wells of CD8+ splenocytes, which are hgp100-tetramer+ (A) or IFN-γ+ (B); bars, SE. *, P < 0.005, compared with corresponding IgG-treated group; ‡, P < 0.005 compared with DTA-1-treated mice without CD4 depletion. Despite supporting the expansion of gp100-specific CD8+ T cells, CD4 depletion abrogated any protection from lethal B16 challenge provided by hgp100 DNA immunization in both groups (data not shown). C, mice (n = 3 per group) were immunized with hgp100 DNA thrice weekly (days 0, 7, and 14) + 1 mg control IgG or DTA-1 injected 1 day before the second immunization (day 6). Groups also received 0.25 mg depleting anti-CD25 antibody (PC61) or control IgG 5 days before the first vaccination. Splenocytes and lymph node cells were harvested 5 days after the final immunization (day 19) and assessed for CD8+ T-cell responses by tetramer assay (C) and intracellular cytokine assay (data not shown). Columns, mean percentage of triplicate wells of CD8+ splenocytes, which are hgp100-tetramer+; bars, SE. *, P < 0.001, compared with PC61/IgG group. D, tumor challenge: mice (n = 15 per group) were immunized as in (C), challenged with 50,000 B16 melanoma cells i.d., and monitored every 2 to 3 days for tumor growth. Kaplan-Meier curves of tumor-free survival. Antibody injected on day −5 is listed first followed by the antibody injected on day 6. Representative of two independent experiments each for (A) to (D).
Similar articles
- Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity.
Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, Miles RC, Sakaguchi S, Houghton AN, van den Brink MR. Ramirez-Montagut T, et al. J Immunol. 2006 Jun 1;176(11):6434-42. doi: 10.4049/jimmunol.176.11.6434. J Immunol. 2006. PMID: 16709800 - Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation.
Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA, Merghoub T, Houghton AN, Wolchok JD. Cohen AD, et al. PLoS One. 2010 May 3;5(5):e10436. doi: 10.1371/journal.pone.0010436. PLoS One. 2010. PMID: 20454651 Free PMC article. - Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T cell responses to tumor-specific antigens.
Côté AL, Zhang P, O'Sullivan JA, Jacobs VL, Clemis CR, Sakaguchi S, Guevara-Patiño JA, Turk MJ. Côté AL, et al. J Immunol. 2011 Jan 1;186(1):275-83. doi: 10.4049/jimmunol.1001308. Epub 2010 Nov 24. J Immunol. 2011. PMID: 21106849 Free PMC article. - Modulation of CTLA-4 and GITR for cancer immunotherapy.
Avogadri F, Yuan J, Yang A, Schaer D, Wolchok JD. Avogadri F, et al. Curr Top Microbiol Immunol. 2011;344:211-44. doi: 10.1007/82_2010_49. Curr Top Microbiol Immunol. 2011. PMID: 20563707 Free PMC article. Review. - CD8+ T cells: GITR matters.
Ronchetti S, Nocentini G, Petrillo MG, Riccardi C. Ronchetti S, et al. ScientificWorldJournal. 2012;2012:308265. doi: 10.1100/2012/308265. Epub 2012 Apr 30. ScientificWorldJournal. 2012. PMID: 22654588 Free PMC article. Review.
Cited by
- Depletion of CD4+CD25+ regulatory T cells enhances interleukin-2-induced antitumor immunity in a mouse model of colon adenocarcinoma.
Imai H, Saio M, Nonaka K, Suwa T, Umemura N, Ouyang GF, Nakagawa J, Tomita H, Osada S, Sugiyama Y, Adachi Y, Takami T. Imai H, et al. Cancer Sci. 2007 Mar;98(3):416-23. doi: 10.1111/j.1349-7006.2006.00385.x. Cancer Sci. 2007. PMID: 17270031 Free PMC article. - Directing dendritic cell immunotherapy towards successful cancer treatment.
Sabado RL, Bhardwaj N. Sabado RL, et al. Immunotherapy. 2010 Jan;2(1):37-56. doi: 10.2217/imt.09.43. Immunotherapy. 2010. PMID: 20473346 Free PMC article. Review. - Glucocorticoid-induced tumor necrosis factor receptor stimulation enhances the multifunctionality of adoptively transferred tumor antigen-specific CD8+ T cells with tumor regression.
Imai N, Ikeda H, Tawara I, Wang L, Wang L, Nishikawa H, Kato T, Shiku H. Imai N, et al. Cancer Sci. 2009 Jul;100(7):1317-25. doi: 10.1111/j.1349-7006.2009.01179.x. Epub 2009 Apr 29. Cancer Sci. 2009. PMID: 19432889 Free PMC article. - Depletion of CD4+ CD25+ regulatory T cells inhibits local tumour growth in a mouse model of B cell lymphoma.
Heier I, Hofgaard PO, Brandtzaeg P, Jahnsen FL, Karlsson M. Heier I, et al. Clin Exp Immunol. 2008 May;152(2):381-7. doi: 10.1111/j.1365-2249.2008.03642.x. Epub 2008 Mar 12. Clin Exp Immunol. 2008. PMID: 18341610 Free PMC article. - Expanding the toolbox of exosome-based modulators of cell functions.
Cheng Q, Dai Z, Shi X, Duan X, Wang Y, Hou T, Zhang Y. Cheng Q, et al. Biomaterials. 2021 Oct;277:121129. doi: 10.1016/j.biomaterials.2021.121129. Epub 2021 Sep 12. Biomaterials. 2021. PMID: 34534861 Free PMC article.
References
- van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R25 CA020449/CA/NCI NIH HHS/United States
- T32 CA009512/CA/NCI NIH HHS/United States
- Z01 BC010763-01/ImNIH/Intramural NIH HHS/United States
- K08CA10260/CA/NCI NIH HHS/United States
- P01 CA033049/CA/NCI NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- CA56821/CA/NCI NIH HHS/United States
- CA33049/CA/NCI NIH HHS/United States
- CA59350/CA/NCI NIH HHS/United States
- CA47179/CA/NCI NIH HHS/United States
- R01 CA056821/CA/NCI NIH HHS/United States
- K08 CA102606/CA/NCI NIH HHS/United States
- P01 CA047179/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials