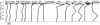Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat - PubMed (original) (raw)
Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat
Ruth E Ley et al. Appl Environ Microbiol. 2006 May.
Abstract
We applied nucleic acid-based molecular methods, combined with estimates of biomass (ATP), pigments, and microelectrode measurements of chemical gradients, to map microbial diversity vertically on a millimeter scale in a hypersaline microbial mat from Guerrero Negro, Baja California Sur, Mexico. To identify the constituents of the mat, small-subunit rRNA genes were amplified by PCR from community genomic DNA extracted from layers, cloned, and sequenced. Bacteria dominated the mat and displayed unexpected and unprecedented diversity. The majority (1,336) of the 1,586 bacterial 16S rRNA sequences generated were unique, representing 752 species (> or =97% rRNA sequence identity) in 42 of the main bacterial phyla, including 15 novel candidate phyla. The diversity of the mat samples differentiated according to the chemical milieu defined by concentrations of O(2) and H(2)S. Bacteria of the phylum Chloroflexi formed the majority of the biomass by percentage of bulk rRNA and of clones in rRNA gene libraries. This result contradicts the general belief that cyanobacteria dominate these communities. Although cyanobacteria constituted a large fraction of the biomass in the upper few millimeters (>80% of the total rRNA and photosynthetic pigments), Chloroflexi sequences were conspicuous throughout the mat. Filamentous Chloroflexi bacteria were identified by fluorescence in situ hybridization within the polysaccharide sheaths of the prominent cyanobacterium Microcoleus chthonoplastes, in addition to free living in the mat. The biological complexity of the mat far exceeds that observed in other polysaccharide-rich microbial ecosystems, such as the human and mouse distal guts, and suggests that positive feedbacks exist between chemical complexity and biological diversity. The sequences determined in this study have been submitted to the GenBank database and assigned accession numbers DQ 329539 to DQ 331020, and DQ 397339 to DQ 397511.
Figures
FIG. 1.
Chemical and biochemical characteristics of the mat as a function of depth. (A) Microelectrode measurements of O2 and H2S concentrations and pH. (B) ATP concentrations. Means of three independent measurements are plotted; the bars show standard errors. (C) Pigment concentrations. For all measurements, October values are plotted; the June values were equivalent (data not shown).
FIG. 2.
Bacterial diversity within the hypersaline microbial mat. (A) Observed and predicted (Chao1, Ace1) numbers of taxa with minimum thresholds ranging from 90 to 100% ID for masked sequences. (B) Collector's curves for taxa (OTUs) with minimum thresholds of 90, 95, 97, 98, 99, and 100% ID.
FIG. 3.
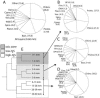
Bacterial diversity in the mat. Proportions of bacterial phyla in the total data set (A), in the oxic zone (0 to 2 mm) (B), in the low-H2S zone (2 to 6 mm) (C), and in the H2S-rich zone (6 to 60 mm) (D) are shown. Others: cyanobacteria, KSB1, OP10, GN03, OP5, GN1, Firmicutes, OP11, GN04, GN05, GN09, GN10, WS1, WS2, GN2, _Deinococcus_-Thermus, GN07, Haloanaerobiales, GN06, GN11, BRC1, OP8, OS-K, GN12, GN13, GN14, actinobacteria, GN15, WS3, GN8, OP9, TM6, and VadinBE97. Abbreviations: Chloro., Chloroflexi; Cyano., Cyanobacteria; Verr., Verrucomicrobia; Planct., Planctomycetales; Spiro., Spirochaetales; Firm., Firmicutes, Bact., Bacteroidetes; Proteo., Proteobacteria. (E) Bacterial community clustering by layer studied (UPGMA tree of UniFrac metric based on 1,585 16S rRNA gene sequences). Shaded areas refer to the different chemical milieus identified by the microelectrode measurements in Fig. 1A.
FIG. 4.
Diagrammatic phylogenetic trees of microbial mat sequences and their cultured and uncultured relatives with associated GenBank accession numbers. Reference sequences of cultured representatives are shown in italics. Wedges represent groups of microbial mat sequences, and single sequences are indicated by their clone names. The length of the top and bottom edges represents the range of sequence divergence. The average depth from which sequences were obtained is indicated next to the wedge, with the total depth range in parentheses. (A) Chloroflexi sequences. Percentages indicate the fraction of Chloroflexi sequences within a given sequence cluster. “Oxic zone” indicates clusters of sequences obtained from surface layers exclusively. (B) Cyanobacterial sequences. Percentages indicate the fraction of cyanobacterial sequences within a given sequence cluster.
FIG. 5.
Depth distributions of the 10 most abundant phyla in the mat. Points indicate the percentage of sequences within each phylum (not the percentage of total sequences) obtained at each depth. The bar indicates 20% of sequences within each group. Shaded areas: see legend to Fig. 2E.
FIG. 6.
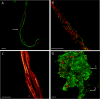
Chloroflexi bacteria and the cyanobacterium M. chthonoplastes in the mat visualized by laser confocal microscopy. (A) Chloroflexi bacteria (red, FISH Chloroflexi probe) entwined with M. chthonoplastes (green, DAPI) at a 1-mm depth. The arrow indicates the edge of the polysaccharide sheath. (B) Chloroflexi bacteria (green, Chloroflexi probe) and M. chthonoplastes (green, autofluorescence of Chl a). (C) Chloroflexi bacteria (thin filaments) and M. chthonoplastes (thick filaments), DAPI stained. (D) Chloroflexi filaments (red, Chloroflexi probe) and polysaccharide material (dull green) at a 50-mm depth. Non-Chloroflexi bacteria are visible as bright green spots (arrow 1). Arrow 2 indicates a buried M. chthonoplastes filament. Scale bars, 10 μm.
FIG. 7.
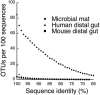
Phylogenetic structure of microbial mat (n = 1,585; this study), human colonic (n = 11,831) (15), and mouse cecal (n = 5,088) (32) 16S rRNA gene sequence data sets. Sequences were clustered into phylotypes based on percent sequence identity (OTUs with similarity thresholds ranging from 65% ID to 100% ID). The ratio of phylotypes at each threshold to the total sequence in each data set is plotted.
Similar articles
- Molecular diversity of fungal and bacterial communities in the marine sponge Dragmacidon reticulatum.
Passarini MR, Miqueletto PB, de Oliveira VM, Sette LD. Passarini MR, et al. J Basic Microbiol. 2015 Feb;55(2):207-20. doi: 10.1002/jobm.201400466. Epub 2014 Sep 11. J Basic Microbiol. 2015. PMID: 25213208 - Novel and unexpected prokaryotic diversity in water and sediments of the alkaline, hypersaline lakes of the Wadi An Natrun, Egypt.
Mesbah NM, Abou-El-Ela SH, Wiegel J. Mesbah NM, et al. Microb Ecol. 2007 Nov;54(4):598-617. doi: 10.1007/s00248-006-9193-y. Epub 2007 Apr 21. Microb Ecol. 2007. PMID: 17450395 - Phylogenetic stratigraphy in the Guerrero Negro hypersaline microbial mat.
Harris JK, Caporaso JG, Walker JJ, Spear JR, Gold NJ, Robertson CE, Hugenholtz P, Goodrich J, McDonald D, Knights D, Marshall P, Tufo H, Knight R, Pace NR. Harris JK, et al. ISME J. 2013 Jan;7(1):50-60. doi: 10.1038/ismej.2012.79. Epub 2012 Jul 26. ISME J. 2013. PMID: 22832344 Free PMC article. - A natural view of microbial biodiversity within hot spring cyanobacterial mat communities.
Ward DM, Ferris MJ, Nold SC, Bateson MM. Ward DM, et al. Microbiol Mol Biol Rev. 1998 Dec;62(4):1353-70. doi: 10.1128/MMBR.62.4.1353-1370.1998. Microbiol Mol Biol Rev. 1998. PMID: 9841675 Free PMC article. Review. - Iron Flocs and the Three Domains: Microbial Interactions in Freshwater Iron Mats.
Brooks CN, Field EK. Brooks CN, et al. mBio. 2020 Dec 15;11(6):e02720-20. doi: 10.1128/mBio.02720-20. mBio. 2020. PMID: 33323508 Free PMC article. Review.
Cited by
- Prokaryotic taxonomy and nomenclature in the age of big sequence data.
Hugenholtz P, Chuvochina M, Oren A, Parks DH, Soo RM. Hugenholtz P, et al. ISME J. 2021 Jul;15(7):1879-1892. doi: 10.1038/s41396-021-00941-x. Epub 2021 Apr 6. ISME J. 2021. PMID: 33824426 Free PMC article. Review. - Culture-independent analysis of indomethacin-induced alterations in the rat gastrointestinal microbiota.
Dalby AB, Frank DN, St Amand AL, Bendele AM, Pace NR. Dalby AB, et al. Appl Environ Microbiol. 2006 Oct;72(10):6707-15. doi: 10.1128/AEM.00378-06. Appl Environ Microbiol. 2006. PMID: 17021222 Free PMC article. - The Vulnerability of Microbial Ecosystems in A Changing Climate: Potential Impact in Shark Bay.
Reinold M, Wong HL, MacLeod FI, Meltzer J, Thompson A, Burns BP. Reinold M, et al. Life (Basel). 2019 Sep 2;9(3):71. doi: 10.3390/life9030071. Life (Basel). 2019. PMID: 31480795 Free PMC article. Review. - Ecophysiology of uncultured filamentous anaerobes belonging to the phylum KSB3 that cause bulking in methanogenic granular sludge.
Yamada T, Kikuchi K, Yamauchi T, Shiraishi K, Ito T, Okabe S, Hiraishi A, Ohashi A, Harada H, Kamagata Y, Nakamura K, Sekiguchi Y. Yamada T, et al. Appl Environ Microbiol. 2011 Mar;77(6):2081-7. doi: 10.1128/AEM.02475-10. Epub 2011 Jan 21. Appl Environ Microbiol. 2011. PMID: 21257808 Free PMC article. - Comparative characterization of the microbial diversities of an artificial microbialite model and a natural stromatolite.
Havemann SA, Foster JS. Havemann SA, et al. Appl Environ Microbiol. 2008 Dec;74(23):7410-21. doi: 10.1128/AEM.01710-08. Epub 2008 Oct 3. Appl Environ Microbiol. 2008. PMID: 18836014 Free PMC article.
References
- Backhed, F., R. E. Ley, J. L. Sonnenburg, D. A. Peterson, and J. I. Gordon. 2005. Host-bacterial mutualism in the human intestine. Science 307:1915-1920. - PubMed
- Bebout, B. M., S. P. Carpenter, D. J. DesMarais, M. Discipulo, T. Embaye, F. Garcia-Pichel, T. M. Hoehler, M. Hogan, L. L. Jahnke, R. M. Keller, S. R. Miller, L. E. Prufert-Bebout, C. Raleigh, M. Rothrock, and K. Turk. 2002. Long-term manipulations of intact microbial mat communities in a greenhouse collaboratory: simulating Earth's present and past field environments. Astrobiology 2:383-402. - PubMed
- Buckley, M. R. 2004. The global genome question: microbes as the key to understanding evolution and ecology. American Academy of Microbiology, Washington, D.C. - PubMed
- Canfield, D. E., and D. J. DesMarais. 1993. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim. Cosmochim. Acta 57:3971-3984. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases