ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency - PubMed (original) (raw)
. 2006 May 17;25(10):2167-77.
doi: 10.1038/sj.emboj.7601115. Epub 2006 May 4.
Sangsoo Kim, Cuiying Xiao, Rui-Hong Wang, Xavier Coumoul, Xiaoyan Wang, Wen Mei Li, Xiao Ling Xu, Joseph A De Soto, Hiroyuki Takai, Sabine Mai, Stephen J Elledge, Noboru Motoyama, Chu-Xia Deng
Affiliations
- PMID: 16675955
- PMCID: PMC1462967
- DOI: 10.1038/sj.emboj.7601115
ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency
Liu Cao et al. EMBO J. 2006.
Abstract
BRCA1 is a checkpoint and DNA damage repair gene that secures genome integrity. We have previously shown that mice lacking full-length Brca1 (Brca1(delta11/delta11)) die during embryonic development. Haploid loss of p53 completely rescues embryonic lethality, and adult Brca1(delta11/delta11)p53+/- mice display cancer susceptibility and premature aging. Here, we show that reduced expression and/or the absence of Chk2 allow Brca1(delta11/delta11) mice to escape from embryonic lethality. Compared to Brca1(delta11/delta11)p53+/- mice, lifespan of Brca1(delta11/delta11)Chk2-/- mice was remarkably extended. Analysis of Brca1(delta11/delta11)Chk2-/- mice revealed that p53-dependent apoptosis and growth defect caused by Brca1 deficiency are significantly attenuated in rapidly proliferating organs. However, in later life, Brca1(delta11/delta11)Chk2-/- female mice developed multiple tumors. Furthermore, haploid loss of ATM also rescued Brca1 deficiency-associated embryonic lethality and premature aging. Thus, in response to Brca1 deficiency, the activation of the ATM-Chk2-p53 signaling pathway contributes to the suppression of neoplastic transformation, while leading to compromised organismal homeostasis. Our data highlight how accurate maintenance of genomic integrity is critical for the suppression of both aging and malignancy, and provide a further link between aging and cancer.
Figures
Figure 1
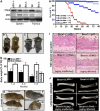
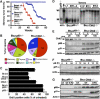
Inactivation of Chk2 rescues embryonic lethality and premature aging caused by Brca1 deficiency. (A) Western blot analysis of spleen and thymus from natural-survived Brca1_Δ_11/Δ_11_ mice for Chk2 and p53 expression (n_=6). (B, C) Photograph of postnatal 1-month-old wild-type, Brca1_Δ_11/Δ_11 Chk2_−/− and Brca1_Δ_11/Δ_11 p53+/− mice. (D) Body weights of Brca1_Δ_11/Δ_11_ Chk2_−/− and Brca1_Δ_11/Δ_11 p53+/− male mice (each time point, n_=10; aging-affected Brca1_Δ_11/Δ_11 Chk2_−/− mice, n_=6). (E) Photograph of 8-month-old Brca1+/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 p53+/− mice. (F, G) Photograph of 8-month-old aging-unaffected (F) and aging-affected (G) Brca1_Δ_11/Δ_11_ _Chk2_−/− mice. An arrow in (G) points to severe kyophosis. (H) Lifespan of Brca1+/Δ11Chk2−/− male and female mice (n_=15), Brca1Δ11/Δ11Chk2−/− female male (n_=30), Brca1Δ11/Δ11Chk2−/− male mice (n_=20) and Brca1Δ11/Δ11p53+/− male mice (n_=14) (F: female; M: Male). (I) Skin histologic section of 8-month-old Brca1+/Δ_11 p53+/−, Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 Chk2_−/− male mice (n_=4). (J) Long-bone radiograph of 8-month-old Brca1+/Δ_11 p53+/−, Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 _Chk2_−/− male mice (_n_=4).
Figure 2
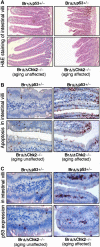
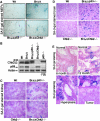
The absence of Chk2 inhibits p53 activity and p53-mediated apoptosis caused by Brca1 deficiency. (A) Small intestine histologic section of 8-month-old Brca1+/Δ_11_ p53+/−, Brca1_Δ_11/Δ_11_ p53+/−, Brca1_Δ_11/Δ_11_ Chk2_−/− (aging unaffected) and Brca1_Δ_11/Δ_11 Chk2_−/− (aging affected) male mice (n_=5). (B) TUNEL-assay on small intestine from 8-month-old Brca1+/Δ_11 p53+/−, Brca1_Δ_11/Δ_11 p53+/−, Brca1_Δ_11/Δ_11_ Chk2_−/− (aging-unaffected) and Brca1_Δ_11/Δ_11 Chk2_−/− (aging affected) male mice (n_=4). (C) p53 expression detected by immunohistochemical staining using an antibody to p53 in small intestine from 8-month-old Brca1+/Δ_11 p53+/−, Brca1_Δ_11/Δ_11 p53+/−, Brca1_Δ_11/Δ_11_ Chk2_−/− (aging-unaffected) and Brca1_Δ_11/Δ_11 _Chk2_−/− (aging-affected) male mice (_n_=4).
Figure 3
Activation of the ATM–Chk2–p53 signaling pathway in response to Brca1 deficiency in embryogenesis. (A–D) γ-H2AX staining on brain tissues of wild-type, Brca1_Δ_11/Δ_11_, Brca1_Δ_11/Δ_11_ Chk2_−/− and Brca1_Δ_11/Δ_11 Atm_−/− E12.5 embryos (n_=3). (E–H) p53-Ser23 staining on brain tissues of wild-type, Brca1_Δ_11/Δ_11, Brca1_Δ_11/Δ_11 Chk2_−/− and Brca1_Δ_11/Δ_11 _Atm_−/− E12.5 embryos (n_=3). (I) Quantification of the γ-H2AX and p53-Ser23-positive cells. (J) Western blot analysis of Atm, Chk2 and p53 expression of passage 1 MEF cells of wild type, Brca1_Δ_11/Δ_11 p53_−/− and Brca1_Δ_11/Δ_11 _Atm_−/− in treated or untreated with γ–irradiation.
Figure 4
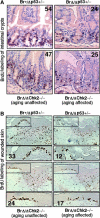
Activation of Chk2–p53 signaling in transit amplifying stem cell proliferation. (A) BrdU-labeling assay for transit amplifying cells of small intestine crypts from 8-month-old Brca1+/Δ_11_ p53+/−, Brca1_Δ_11/Δ_11_ p53+/−, Brca1_Δ_11/Δ_11_ Chk2_−/− (aging unaffected) and Brca1_Δ_11/Δ_11 Chk2_−/− (aging affected) male mice (n_=4). (B) BrdU-labeling assay for transit amplifying cells of skin keratinocyte from 8-month-old Brca1+/Δ_11 p53+/−, Brca1_Δ_11/Δ_11 p53+/−, Brca1_Δ_11/Δ_11_ Chk2_−/− (aging unaffected) and Brca1_Δ_11/Δ_11 _Chk2_−/− (aging affected) male mice after wounded 3 days (_n_=4). Number of BrdU-positive cells in these areas were counted and shown in each panel.
Figure 5
Incidence, latency, spectrum and p53 status in tumors from Brca1_Δ_11/Δ_11_ p53+/− and Brca1_Δ_11/Δ_11_ Chk2_−/− female mice. (A) Onset of Brca1-associated mammary tumors of Brca1+/Δ_11 Chk2_−/−, Brca1_Δ_11/Δ_11 Chk2_−/− and Brca1_Δ_11/Δ_11 p53+/− female mice. (B) Tumor spectra of Brca1_Δ_11/Δ_11_ p53+/− and Brca1_Δ_11/Δ_11_ Chk2_−/− female mice. (C) Analysis of the G1/S checkpoint of passage 1 wild-type, Brca1_Δ_11/Δ_111, Brca1_Δ_11/Δ_11_ Chk2_−/−, Chk2_−/−, Brca1_Δ_11/Δ_11 p53_−/− and p53_−/− MEF cells (n_=3) upon 10 Gy γ-IR. The percentage of BrdU-positive cells after γ-IR relative to the unirradiated controls was shown. (The profiles of FACS were shown in Supplementary Figure 3B and C.) (D) Southern blot analysis of p53 on breast tumors (BT) and lymphomas (L) from Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 Chk2_−/− mice by Eco_RI digestion. Wild-type (Wt) band (16 kb) and mutant (Mt) bands (8 kb) were indicated. (E) Western blot analysis for p53 and p21 of cell lines derived from Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 Chk2_−/− mice mammary tumors without (UT) and with 10 Gy γ-IR after 6 h. (F) Time point of p21 expression of tumor cell lines from Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 Chk2_−/− mice after 10 Gy of γ-IR. (G) Western blot analysis of p53 and p21 expression of tumor cell lines from Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 _Chk2_−/− mice by without (UT), 100 J/m2 of UV-irradiation and 50 μg/ml of 5-fluorouracil (5-FU) treatment for 6 h.
Figure 6
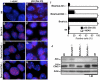
Senescence in aging and tumorigenesis. (A) SA-β-gal staining of passage 4 MEF cells from wild-type, Brca1_Δ_11/Δ_11_, Brca1_Δ_11/Δ_11_ p53_−/− and Brca1_Δ_11/Δ_11 Chk2_−/− E14.5 embryos. (B) Western blot analysis for Chk2 and p53 expression of passage 4 MEF cells from wild-type, Brca1_Δ_11/Δ_11, Brca1_Δ_11/Δ_11_ p53_−/− and Brca1_Δ_11/Δ_11 Chk2_−/− E14.5 embryos. (C) SA-β-gal staining of kidney from 8-month-old wild-type, Chk2_−/−, Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 Chk2_−/− mice (aging affected). (D) SA-β-gal staining of brain from 8-month-old wild-type, Chk2_−/−, Brca1_Δ_11/Δ_11 p53+/− and Brca1_Δ_11/Δ_11 Chk2_−/− mice (aging affected). (E) SA-β-gal staining of normal and hyperplasia mammary tissue, and mammary tumor Brca1_Δ_11/Δ_11 _Chk2_−/− female mice.
Figure 7
Model of the ATM–Chk2–p53 signaling pathway upon Brca1 mutation-associated premature aging and tumorigenesis. ATM–Chk2–p53 signaling pathway senses DNA damage/genomic instability and acts as a gatekeeper to eliminate mutations, but, as a side effect, it may also lead to premature aging.
References
- Bachelier R, Xu X, Wang X, Li W, Naramura M, Gu H, Deng CX (2003) Normal lymphocyte development and thymic lymphoma formation in Brca1 exon-11-deficient mice. Oncogene 22: 528–537 - PubMed
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171 - PubMed
- Bartek J, Lukas J (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–429 - PubMed
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434: 864–870 - PubMed
- Bell DW, Varley JM, Szydlo TE, Kang DH, Wahrer DC, Shannon KE, Lubratovich M, Verselis SJ, Isselbacher KJ, Fraumeni JF, Birch JM, Li FP, Garber JE, Haber DA (1999) Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286: 2528–2531 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous