The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium - PubMed (original) (raw)
Review
The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium
Samantha R Oakes et al. Breast Cancer Res. 2006.
Abstract
Massive tissue remodelling occurs within the mammary gland during pregnancy, resulting in the formation of lobuloalveoli that are capable of milk secretion. Endocrine signals generated predominantly by prolactin and progesterone operate the alveolar switch to initiate these developmental events. Here we review the current understanding of the components of the alveolar switch and conclude with an examination of the role of the ets transcription factor Elf5. We propose that Elf5 is a key regulator of the alveolar switch.
Figures
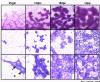
Figure 1
Alveolar morphogenesis. Mammary wholemounts (Carmine alum stain top row) and mammary cellular architecture (low power, middle row; high power, bottom row) in virgin, 12 days posts coitus (dpc), 18 dpc and 1 day post partum (1 dpp) murine mammary glands. Ductal epithelial cells (arrow) and myoepithelial cells (arrowhead) arise from a common mammary epithelial stem cell. Massive epithelial cell proliferation occurs at the onset of pregnancy, which is co-ordinated predominantly by prolactin and progesterone. At mid-pregnancy (12 dpc), developing alveoli continue to proliferate and polarise to form a sphere-like single layer of epithelial cells enveloping a circular lumen (indicated by X). This is followed by further cell proliferation and differentiation categorised by the expression of milk genes and the formation of cytoplasmic lipid droplets (indicated by asterisks). At 18 dpc, alveoli have large amounts of lipid and milk protein expression is increased. At parturition, tight junctions between alveolar cells close and milk proteins and lipid are secreted into the alveolar lumen (X). An expansion of the vasculature (open arrows) and reduction in the adipocyte (A) area is also apparent in the stroma.
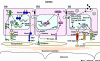
Figure 2
Molecular control of alveolar morphogenesis. Signalling from the progesterone receptor (Pgr) and prolactin receptor (Prlr) is essential for alveolar morphogenesis in pregnancy. Increases in serum progesterone (Pg) and prolactin (Prl) result in luminal cell proliferation during early pregnancy, which continues throughout gestation. (a,b) Heterogenous receptor patterning is essential for complete alveolar morphogenesis. (a) Transforming growth factor (Tgf)-β1 signalling via phosphorylation of Smad results in the transcription of target genes, which act to control proliferation in steroid receptor positive cells. Wnt4 and RankL are transcribed in response to Pgr signalling, probably in cooperation with Prl signalling, and appear to stimulate proliferation of neighbouring cells via paracrine mechanisms. (b) RankL binds to its receptor Rank in a neighbouring cell and activates the RankL/nuclear factor (NF)-κB pathway, resulting in cyclin-D1 (Ccnd1) transcription and proliferation. Wnt4 binds and activates its target β-catenin, which has specific roles for both luminal and myo-epithelium for cell fate decisions involving both proliferation and differentiation. (a,c) Prl binds to Prlr and activates the Jak2/Stat5 cascade, resulting in the transcription of genes, including various transcription factors (TF) involved in epithelial morphogenesis and branching (Wnt4), establishment of epithelial polarity and cell-cell interactions (claudins and connexins), stromal epithelial interactions (collagen and laminin), proteins that regulate their own pathway (Socs1/2) and lactation (serotonin and milk proteins). Prl signalling also results in the transcription of cyclin D1 via an insulin growth factor 2 dependent mechanism. The ets transcription factor Elf5, transcribed in response to Prl, can completely compensate for the loss of Prlr signalling. Laminin in the extracellular matrix binds to β1-integrin when contact between the basement membrane and the luminal epithelium is established, and is essential for the maintenance of alveolar cell polarity and differentiation. ErbB4 and its ligands complement Prlr signalling as activation of ErbB4 results in Stat5 phosphorylation and translocation to the nucleus. GJ, gap junction; L, lipid droplet; TJ, tight junction.
Similar articles
- Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - A Blog-Based Study of Autistic Adults' Experiences of Aloneness and Connection and the Interplay with Well-Being: Corpus-Based and Thematic Analyses.
Petty S, Allen S, Pickup H, Woodier B. Petty S, et al. Autism Adulthood. 2023 Dec 1;5(4):437-449. doi: 10.1089/aut.2022.0073. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116056 Free PMC article. - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- Maternal chitosan oligosaccharide intervention optimizes the production performance and health status of gilts and their offspring.
Ho T, Jahan M, Haque Z, Kracht S, Wynn PC, Du Y, Gunn A, Wang B. Ho T, et al. Anim Nutr. 2020 Jun;6(2):134-142. doi: 10.1016/j.aninu.2020.02.001. Epub 2020 Mar 5. Anim Nutr. 2020. PMID: 32542193 Free PMC article. - Periconceptional and Prenatal Exposure to Metals and Extracellular Vesicle and Particle miRNAs in Human Milk: A Pilot Study.
Howe CG, Armstrong DA, Muse ME, Gilbert-Diamond D, Gui J, Hoen AG, Palys TJ, Barnaby RL, Stanton BA, Jackson BP, Christensen BC, Karagas MR. Howe CG, et al. Expo Health. 2023 Dec;15(4):731-743. doi: 10.1007/s12403-022-00520-1. Epub 2022 Nov 3. Expo Health. 2023. PMID: 38074282 Free PMC article. - Regulation of DCIS to invasive breast cancer progression by Singleminded-2s (SIM2s).
Scribner KC, Behbod F, Porter WW. Scribner KC, et al. Oncogene. 2013 May 23;32(21):2631-9. doi: 10.1038/onc.2012.286. Epub 2012 Jul 9. Oncogene. 2013. PMID: 22777354 Free PMC article. - TNFAIP3 Reduction-of-Function Drives Female Infertility and CNS Inflammation.
Zammit NW, McDowell J, Warren J, Muskovic W, Gamble J, Shi YC, Kaczorowski D, Chan CL, Powell J, Ormandy C, Brown D, Oakes SR, Grey ST. Zammit NW, et al. Front Immunol. 2022 Apr 8;13:811525. doi: 10.3389/fimmu.2022.811525. eCollection 2022. Front Immunol. 2022. PMID: 35464428 Free PMC article. - Cell-matrix interactions in mammary gland development and breast cancer.
Muschler J, Streuli CH. Muschler J, et al. Cold Spring Harb Perspect Biol. 2010 Oct;2(10):a003202. doi: 10.1101/cshperspect.a003202. Epub 2010 Aug 11. Cold Spring Harb Perspect Biol. 2010. PMID: 20702598 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical