Eosinophil activity in Schistosoma mansoni infections in vivo and in vitro in relation to plasma cytokine profile pre- and posttreatment with praziquantel - PubMed (original) (raw)
Eosinophil activity in Schistosoma mansoni infections in vivo and in vitro in relation to plasma cytokine profile pre- and posttreatment with praziquantel
Claus M Reimert et al. Clin Vaccine Immunol. 2006 May.
Abstract
Eosinophil activity in vivo and in vitro was studied in relation to infection intensities and plasma cytokine profiles of 51 Schistosoma mansoni-infected Ugandan fishermen before treatment and 24 h and 3 weeks posttreatment. Blood eosinophil numbers significantly declined 24 h posttreatment, but significant eosinophilia had developed by 3 weeks posttreatment. Cellular eosinophil cationic protein (ECP) content increased significantly during the transient eosinopenia but was significantly reduced 3 weeks later. No similar reduction in cellular eosinophil protein X (EPX) content was seen. Before treatment, S. mansoni infection intensity was positively correlated with 24-h boosts in plasma interleukin-5 (IL-5) and IL-6 levels, which were in turn negatively correlated with the posttreatment fall in eosinophil numbers. Significant correlations were observed between pretreatment infection intensities and plasma IL-10 and eotaxin levels. Treatment induced significant fluctuations in plasma IL-5, IL-6, IL-10, tumor necrosis factor alpha (TNF-alpha), and eotaxin levels. Optimal relative release of ECP and EPX in vitro was detected in S. mansoni soluble egg antigen-stimulated cultures during transient eosinopenia. Our data suggest that blood eosinophils are activated during S. mansoni infection and that treatment induces a burst in released antigens, causing increased production of IL-5, IL-6, IL-10, and eotaxin; a drop in TNF-alpha levels; and a transient sequestration of eosinophils, which leaves fewer degranulated eosinophils in the circulation 24 h posttreatment, followed by the development of eosinophilia 3 weeks later. During these events, it appears that preferential release of ECP occurs in vivo. Moreover, it is possible that infection intensity-dependent levels of plasma IL-10 may be involved in the prevention of treatment-induced anaphylactic reactions.
Figures
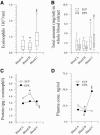
FIG. 1.
Pre- and posttreatment blood eosinophil counts, total ECP counts, and total EPX levels in whole blood extracts; cellular content of ECP and EPX; and plasma ECP and EPX levels. The boxes in panels A and B represent the 25th, 50th, and 75th percentile ranges, and the error bars show the ranges of 10th and 90th percentiles. (A) Blood eosinophil counts. *, P = 0.04; §, P = 1.5 × 10−6. (B) Total ECP and EPX counts in whole blood extracts. *, P ≤ 1.0 × 10−12; §, P = 1.0 × 10−8. (C) Cellular content of ECP and EPX (medians). *, P = 0.005; §, P = 0.002. No significant fluctuations in the cellular content of EPX were seen. (D) Plasma ECP and EPX levels (medians). *, P = 0.001; #, P = 0.019; §, P ≤ 0.027.
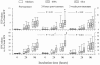
FIG. 2.
Relative release in vitro of ECP and EPX in whole blood cultures stimulated with SWA, SEA, or medium alone. The relative release is calculated as the released amount of ECP and EPX as a percentage of the total content of ECP or EPX extracted from whole blood before the cultures were set up. The boxes represent the 25th, 50th, and 75th percentile ranges, and the error bars illustrate the ranges of the 10th and 90th percentiles. Significant time-dependent release of ECP and EPX was seen in all cultures (P ≤ 2.2 × 10−15 for all). Optimal release of both ECP and EPX was seen in SEA-stimulated cultures from bleed B after 96 h of incubation. For optimal ECP release, the P values were 3.8 × 10−6 and 1.6 × 10−7 (*), respectively, compared to 96-h cultures stimulated with SWA or medium alone. For optimal EPX release, P values were 7.34 × 10−10 and 5.46 × 10−10 (§), compared to 96-h cultures from bleed B stimulated with SWA or medium alone. Levels of significance compared to SEA-stimulated 96-h cultures from bleed A and bleed C are indicated.
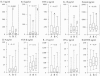
FIG. 3.
Plasma cytokine levels before treatment (bleed A) and 24 h (bleed B) and 3 weeks (bleed C) after treatment. The boxes represent the 25th, 50th, and 75th percentile ranges, and the error bars illustrate the ranges of the 10th and 90th percentiles. Significant fluctuations as tested by the Friedman ρ test (levels of significance are indicated in the figures) were seen in IL-5, IL-6, TNF-α, IL-10, and eotaxin-1 levels. No significant fluctuations were seen in IL-4, TGF-β, IL-13, IFN-γ, or RANTES levels. For IL-5, a significant increase (bleed A versus bleed B) followed by a significant decline (bleed B versus bleed C) (* and §, P ≤ 5.0 × 10−6) was seen; in addition, a significant decline (P = 0.02) was seen when bleed A was compared with bleed C. For IL-6, a nonsignificant increase (*, P = 0.063), followed by a significant decline (bleed B versus bleed C) (§, P = 0.016), was seen. For TNF-α, a significant decline (*, P = 0.001) was seen. For IL-10, a significant increase (*, P = 0.025) followed by a significant decline (bleed B versus bleed C) (§, P = 0.033) was seen. For eotaxin, a significant decline (bleed B versus bleed C) (*, P = 0.026) was seen.
FIG. 4.
Plasma cytokine levels before treatment (bleed A) and 24 h posttreatment (bleed B) in high-eosinophil responders (n = 33; open boxes) and low-eosinophil responders (n = 18; hatched boxes). The boxes represent the 25th, 50th, and 75th percentile ranges, and the error bars show the ranges of the 10th and 90th percentiles.
Similar articles
- Assessment of Schistosoma mansoni induced intestinal inflammation by means of eosinophil cationic protein, eosinophil protein X and myeloperoxidase before and after treatment with praziquantel.
Reimert CM, Tukahebwa EM, Kabatereine NB, Dunne DW, Vennervald BJ. Reimert CM, et al. Acta Trop. 2008 Mar;105(3):253-9. doi: 10.1016/j.actatropica.2007.11.004. Epub 2007 Nov 29. Acta Trop. 2008. PMID: 18177822 - Changes in IgE- and antigen-dependent histamine-release in peripheral blood of Schistosoma mansoni-infected Ugandan fishermen after treatment with praziquantel.
Satti MZ, Cahen P, Skov PS, Joseph S, Jones FM, Fitzsimmons C, Hoffmann KF, Reimert C, Kariuki HC, Kazibwe F, Mwatha JK, Kimani G, Vennervald BJ, Ouma JH, Kabatereine NB, Dunne DW. Satti MZ, et al. BMC Immunol. 2004 Apr 21;5:6. doi: 10.1186/1471-2172-5-6. BMC Immunol. 2004. PMID: 15102330 Free PMC article. - Rapidly boosted Plasma IL-5 induced by treatment of human Schistosomiasis haematobium is dependent on antigen dose, IgE and eosinophils.
Wilson S, Jones FM, Fofana HK, Doucouré A, Landouré A, Kimani G, Mwatha JK, Sacko M, Vennervald BJ, Dunne DW. Wilson S, et al. PLoS Negl Trop Dis. 2013;7(3):e2149. doi: 10.1371/journal.pntd.0002149. Epub 2013 Mar 28. PLoS Negl Trop Dis. 2013. PMID: 23556029 Free PMC article. - Posttreatment changes in cytokines induced by Schistosoma mansoni egg and worm antigens: dissociation of immunity- and morbidity-associated type 2 responses.
Wilson S, Jones FM, Kenty LC, Mwatha JK, Kimani G, Kariuki HC, Dunne DW. Wilson S, et al. J Infect Dis. 2014 Jun 1;209(11):1792-800. doi: 10.1093/infdis/jit826. Epub 2013 Dec 19. J Infect Dis. 2014. PMID: 24357629 Free PMC article. - Eosinophil activation status, cytokines and liver fibrosis in Schistosoma mansoni infected patients.
Silveira-Lemos D, Teixeira-Carvalho A, Martins-Filho OA, Alves Oliveira LF, Costa-Silva MF, Matoso LF, de Souza LJ, Gazzinelli A, Corrêa-Oliveira R. Silveira-Lemos D, et al. Acta Trop. 2008 Nov-Dec;108(2-3):150-9. doi: 10.1016/j.actatropica.2008.04.006. Epub 2008 Apr 15. Acta Trop. 2008. PMID: 18550021
Cited by
- Distinguishment of parasite-infected children from pediatric inpatients with both eosinophilia and effusion.
Miao R, Zhu Y, Wang Z, Luo S, Wan C. Miao R, et al. Medicine (Baltimore). 2020 Apr;99(14):e19625. doi: 10.1097/MD.0000000000019625. Medicine (Baltimore). 2020. PMID: 32243388 Free PMC article. - Immuno-evasive tactics by schistosomes identify an effective allergy preventative.
Griffith Q, Liang Y, Whitworth P, Rodriguez-Russo C, Gul A, Siddiqui AA, Connor J, Mwinzi P, Ganley-Leal L. Griffith Q, et al. Exp Parasitol. 2015 Jun;153:139-50. doi: 10.1016/j.exppara.2015.03.012. Epub 2015 Mar 24. Exp Parasitol. 2015. PMID: 25819297 Free PMC article. - Levels of serum eosinophil cationic protein are associated with hookworm infection and intensity in endemic communities in Ghana.
Amoani B, Adu B, Frempong MT, Sarkodie-Addo T, Nuvor SV, Wilson MD, Gyan B. Amoani B, et al. PLoS One. 2019 Sep 12;14(9):e0222382. doi: 10.1371/journal.pone.0222382. eCollection 2019. PLoS One. 2019. PMID: 31513658 Free PMC article. - Elevated Systemic Levels of Eosinophil, Neutrophil, and Mast Cell Granular Proteins in Strongyloides Stercoralis Infection that Diminish following Treatment.
Rajamanickam A, Munisankar S, Bhootra Y, Dolla CK, Nutman TB, Babu S. Rajamanickam A, et al. Front Immunol. 2018 Feb 9;9:207. doi: 10.3389/fimmu.2018.00207. eCollection 2018. Front Immunol. 2018. PMID: 29479356 Free PMC article. - Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection.
Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW. Wilson S, et al. Infect Immun. 2008 May;76(5):2212-8. doi: 10.1128/IAI.01433-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285496 Free PMC article.
References
- Ackerman, S. J., G. M. Kephart, F. H. Francis, K. Awadzi, G. J. Geich, and E. A. Ottesen. 1990. Eosinophil degranulation. An immunologic determinant in the pathogenesis of the Mazzotti reaction in human onchocerciasis. J. Immunol. 144:3961-3969. - PubMed
- Asadullah, K., W. Sterry, and H. D. Volk. 2003. Interleukin-10 therapy—review of a new approach. Pharmacol. Rev. 55:241-269. - PubMed
- Berhe, N., S. G. Gundersen, F. Abebe, H. Birrie, G. Medhin, and T. Gemetchu. 1999. Praziquantel side effects and efficacy related to Schistosoma mansoni egg loads and morbidity in primary school children in north-east Ethiopia. Acta Trop. 72:53-63. - PubMed
- Booij-Noord, H., K. de Vries, H. J. Sluiter, and N. G. M. Orie. 1972. Late bronchial obstructive reaction to experimental inhalation of house dust extract. Clin. Allergy 2:43-61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous