Neurons produce type I interferon during viral encephalitis - PubMed (original) (raw)
Neurons produce type I interferon during viral encephalitis
Sophie Delhaye et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Type I interferons, also referred to as IFN-alpha/beta, form the first line of defense against viral infections. Major IFN-alpha/beta producers in the periphery are the plasmacytoid dendritic cells (pDCs). Constitutive expression of the IFN regulatory factor (IRF)-7 enables pDCs to rapidly synthesize large amounts of IFN-alpha/beta after viral infection. In the central nervous system (CNS), pDCs are considered to be absent from the parenchyma, and little is known about the cells producing IFN-alpha/beta. The study presented here aimed to identify the cells producing IFN-alpha/beta in the CNS in vivo after infection by neurotropic viruses such as Theiler's virus and La Crosse virus. No cells with high constitutive expression of IRF-7 were detected in the CNS of uninfected mice, suggesting the absence of cells equivalent to pDCs. Upon viral infection, IFN-beta and some subtypes of IFN-alpha, but not IFN-epsilon or IFN-kappa, were transcriptionally up-regulated. IFN-alpha/beta was predominantly produced by scattered parenchymal cells and much less by cells of inflammatory foci. Interestingly, in addition to some macrophages and ependymal cells, neurons turned out to be important producers of both IFN-alpha and IFN-beta. However, only 3% of the infected neurons produced IFN-alpha/beta, suggesting that some restriction to IFN-alpha/beta production existed in these cells. All CNS cell types analyzed, including neurons, were able to respond to type I IFN by producing Mx or IRF-7. Our data show that, in vivo, neurons take an active part to the antiviral defense by being both IFN-alpha/beta producers and responders.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
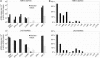
Fig. 1.
Type I IFNs and IRF-7 expression in brains of mice infected with TMEV(GDVII) or LACVdelNSs. Total RNA was extracted from brains of uninfected animals (FVB mice), mice infected for 5 days with TMEV(GDVII) (FVB mice), or mice infected for 7 days with LACVdelNSs (B6.A2G-Mx1 mice). (A) Quantitative RT-PCR was performed to quantify total IFN-α, IFN-α4, IFN-β, limitin, IFN-κ, IFN-ε, and IRF-7 transcripts. The results are expressed as cDNA copy numbers per 104 copies of β-actin cDNA. (B) Expression profile of IFN-α subtypes. IFN-α was amplified by RT-PCR, by using a mix of primers amplifying all IFN-α subtypes. PCR products from two independent experiments were subcloned and sequenced to identify the IFN-α subtypes expressed. For TMEV(GDVII) and LACVdelNSs, 77 and 100 individual clones, respectively, were analyzed.
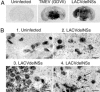
Fig. 2.
IRF-7 mRNA detection in infected and uninfected mouse brains. ISH with an IRF-7-specific probe was performed on mouse brain sections from uninfected and TMEV(GDVII)-infected FVB mice or from LACVdelNSs-infected B6.A2G-Mx1 mice. (A) Macroscopic analysis showing absence of IRF-7-expressing areas in the CNS of uninfected mice and strong up-regulation of IRF-7 transcription in infected mice. (B) Microscopic analysis: IRF-7 mRNA was not detected in uninfected mice (1, thalamic area). IRF-7 was clearly detectable in most cells of LACVdelNSs-infected areas: thalamus (2) and hippocampal neurons (3). IRF-7 up-regulation was slightly more prominent in some inflammatory cells (arrows) (4). (Scale bar: 10 μm.)
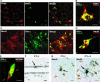
Fig. 3.
Localization of IFN-α/β-expressing cells in the brain of mice infected by neurotropic viruses. ISH with IFN-α4 or IFN-β probes were performed on brain sections from uninfected, TMEV(DA)-infected, or TMEV(GDVII)-infected FVB mice. B6.A2G-Mx1 mice were infected with LACVdelNSs. (A) Macroscopic analysis. (B) Microscopic analysis of TMEV(GDVII)-infected mice showing, on adjacent sections, colocalization of areas with viral RNA, IFN-α, and IFN-β transcripts. (Scale bar: 100 μm.) (C) ISH with a probe for IFN-β and toluidine blue coloration, in an inflamed brain area. (Scale bar: 10 μm.) The cell positive for IFN-β (arrow) is out of the inflammatory focus (arrowhead).
Fig. 4.
Identification of IFN-α-producing cells in brain sections of infected mice. (A_–_E) Confocal microscopy. (Scale bar: 10 μm.) (A, B, D, and E) TMEV(GDVII)-infected FVB mice. (A) Colocalization of viral TMEV antigen staining and IFN-α staining. (B) Higher magnification of double TMEV (red)/IFN-α (green) staining. (C) TMEV(GDVII)-infected SJL mice. Colocalization of a neuronal marker (NeuN) and IFN-α staining in the region of the hippocampus. (D) High magnification of double NeuN (red)/IFN-α (green) staining. (E) Macrophages, detected by a MOMA2 staining (red), can also produce IFN-α (green). (F) ISH with IFN-β probes were performed on brain sections from LACVdelNSs-infected mice. Epithelial cell positive for IFN-β (arrow). (G) Immunohistochemistry of neurons (NeuN, in brown) in combination with ISH with IFN-α5 or IFN-β probes. Brain sections are from B6.A2G-Mx1 mice infected with LACVdelNSs.
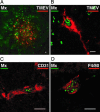
Fig. 5.
Identification of IFN-α-responding cells in brain sections of infected mice. (A_–_D) Expression of Mx1 in TMEV(GDVII)-infected BALB.A2G-Mx1 mice (confocal microscopy). Mx1 staining appears in green as a nuclear dotted pattern. (Scale bars: 10 μm.) (A and B) Viral TMEV antigen (red). (C) Endothelial cell detected by a CD31 staining (red). (D) Macrophage detected by a F4/80 staining (red).
Similar articles
- Ifit2 deficiency results in uncontrolled neurotropic coronavirus replication and enhanced encephalitis via impaired alpha/beta interferon induction in macrophages.
Butchi NB, Hinton DR, Stohlman SA, Kapil P, Fensterl V, Sen GC, Bergmann CC. Butchi NB, et al. J Virol. 2014 Jan;88(2):1051-64. doi: 10.1128/JVI.02272-13. Epub 2013 Nov 6. J Virol. 2014. PMID: 24198415 Free PMC article. - Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection.
Daffis S, Samuel MA, Suthar MS, Keller BC, Gale M Jr, Diamond MS. Daffis S, et al. J Virol. 2008 Sep;82(17):8465-75. doi: 10.1128/JVI.00918-08. Epub 2008 Jun 18. J Virol. 2008. PMID: 18562536 Free PMC article. - Alpha/Beta Interferon (IFN-α/β) Signaling in Astrocytes Mediates Protection against Viral Encephalomyelitis and Regulates IFN-γ-Dependent Responses.
Hwang M, Bergmann CC. Hwang M, et al. J Virol. 2018 Apr 27;92(10):e01901-17. doi: 10.1128/JVI.01901-17. Print 2018 May 15. J Virol. 2018. PMID: 29491163 Free PMC article. - Distinct functions of IRF-3 and IRF-7 in IFN-alpha gene regulation and control of anti-tumor activity in primary macrophages.
Solis M, Goubau D, Romieu-Mourez R, Genin P, Civas A, Hiscott J. Solis M, et al. Biochem Pharmacol. 2006 Nov 30;72(11):1469-76. doi: 10.1016/j.bcp.2006.06.002. Epub 2006 Jul 17. Biochem Pharmacol. 2006. PMID: 16846591 Review. - The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors.
Taniguchi T, Takaoka A. Taniguchi T, et al. Curr Opin Immunol. 2002 Feb;14(1):111-6. doi: 10.1016/s0952-7915(01)00305-3. Curr Opin Immunol. 2002. PMID: 11790540 Review.
Cited by
- Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice.
Kallfass C, Ackerman A, Lienenklaus S, Weiss S, Heimrich B, Staeheli P. Kallfass C, et al. J Virol. 2012 Oct;86(20):11223-30. doi: 10.1128/JVI.01093-12. Epub 2012 Aug 8. J Virol. 2012. PMID: 22875966 Free PMC article. - Extended JAK activation and delayed STAT1 dephosphorylation contribute to the distinct signaling profile of CNS neurons exposed to interferon-gamma.
Podolsky MA, Solomos AC, Durso LC, Evans SM, Rall GF, Rose RW. Podolsky MA, et al. J Neuroimmunol. 2012 Oct 15;251(1-2):33-8. doi: 10.1016/j.jneuroim.2012.06.006. Epub 2012 Jul 4. J Neuroimmunol. 2012. PMID: 22769061 Free PMC article. - Cytomegalovirus induces interferon-stimulated gene expression and is attenuated by interferon in the developing brain.
van den Pol AN, Robek MD, Ghosh PK, Ozduman K, Bandi P, Whim MD, Wollmann G. van den Pol AN, et al. J Virol. 2007 Jan;81(1):332-48. doi: 10.1128/JVI.01592-06. Epub 2006 Oct 25. J Virol. 2007. PMID: 17065212 Free PMC article. - QKI-7 regulates expression of interferon-related genes in human astrocyte glioma cells.
Jiang L, Saetre P, Radomska KJ, Jazin E, Lindholm Carlström E. Jiang L, et al. PLoS One. 2010 Sep 29;5(9):e13079. doi: 10.1371/journal.pone.0013079. PLoS One. 2010. PMID: 20927331 Free PMC article. - Long-distance interferon signaling within the brain blocks virus spread.
van den Pol AN, Ding S, Robek MD. van den Pol AN, et al. J Virol. 2014 Apr;88(7):3695-704. doi: 10.1128/JVI.03509-13. Epub 2014 Jan 15. J Virol. 2014. PMID: 24429359 Free PMC article.
References
- Honda K., Yanai H., Takaoka A., Taniguchi T. Int. Immunol. 2005;17:1367–1378. - PubMed
- Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. Nat. Immunol. 2003;4:491–496. - PubMed
- Sharma S., tenOever B. R., Grandvaux N., Zhou G. P., Lin R., Hiscott J. Science. 2003;300:1148–1151. - PubMed
- Siegal F. P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P. A., Shah K., Ho S., Antonenko S., Liu Y. J. Science. 1999;284:1835–1837. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous