Development of NG2 neural progenitor cells requires Olig gene function - PubMed (original) (raw)
Development of NG2 neural progenitor cells requires Olig gene function
Keith L Ligon et al. Proc Natl Acad Sci U S A. 2006.
Abstract
In the adult central nervous system, two distinct populations of glial cells expressing the chondroitin sulfate proteoglycan NG2 have been described: bipolar progenitor cells and more differentiated "synantocytes." These cells have diverse neurological functions, including critical roles in synaptic transmission, repair, and regeneration. Despite their potential importance, the genetic factors that regulate NG2 cell development are poorly understood, and the relationship of synantocytes to the oligodendroglial lineage, in particular, remains controversial. Here, we show that >90% of embryonic and adult NG2 cells express Olig2, a basic helix-loop-helix transcription factor required for oligodendrocyte lineage specification. Analysis of mice lacking Olig function demonstrates a failure of NG2 cell development at embryonic and perinatal stages that can be rescued by addition of a transgene containing the human OLIG2 locus. These findings show a general requirement for Olig function in NG2 cell development and highlight further roles for Olig transcription factors in neural progenitor cells.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
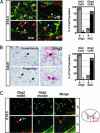
NG2 cells express Olig2 in mouse brain. (A) Deconvolution images of immunofluorescent staining showing NG2 (green Cy5) and Olig2 (red Cy2) are coexpressed in neural cells with NG2 synantocyte and progenitor morphology (white arrowheads) in normal 18.5 dpc mouse neocortex (cx), ventral forebrain (vfb), and developing white matter (iz). Olig2+ cells (white arrowheads) in the subventricular zone (svz) rarely expressed NG2. NG2 staining in pericytes around blood vessels (white arrow in svz) served as internal control. Graph shows summed results of manual cell counting at 18.5 dpc (2,949 total cells counted; n = 3 animals). (B) Double IHC staining for NG2 (red, alkaline phosphatase) and Olig2 [brown, diaminobenzidine (DAB)] in adult rat brain. Colocalization was seen in cells with complex synantocyte morphology (Upper) and cells with progenitor morphology (Lower) occurring as pairs of recently divided cells. Graph shows summed results of manual cell counting from adult mouse brain (578 total cells counted; n = 2 animals). The percentage of Olig2+ NG2 cells was >98% in all subregions of the adult brain. Sections are hematoxylin counterstained. (C) Specificity testing of Olig2 mAb TV37–1C10 in spinal cord white matter (red box in diagram) of Olig2+/− mouse at 18.5 dpc shows precise colocalization of signal from established rabbit polyclonal anti-Olig2 antibody DF308 (red) with that from the mouse anti-Olig2 antibody TV37–1C10 (green). Similar findings were obtained at embryonic and adult stages in brain (data not shown). Absence of any specific labeling in _Olig2_−/− mutant spinal cord (Lower) confirms specificity for Olig2. Arrowhead marks autofluorescence in blood vessels (bv).
Fig. 2.
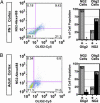
Flow cytometry characterization of NG2 and Olig2 cell populations in postnatal mouse brain. (A) Flow analysis of full thickness dorsal cortical mantle from postnatal day 1 (PN1) mouse for NG2 (rabbit polyclonal; Chemicon) and Olig2 (mouse monoclonal, TV37–1C10) (n = 2 animals). (B) Flow analysis of adult mouse dorsal neocortex for NG2 (rabbit polyclonal; Chemicon) and Olig2 (mouse monoclonal TV37–1C10) (n = 2 animals). Note that, at both stages, >90% of NG2 cells are Olig2+. Conversely, NG2 cells represent <31% of the total Olig2+ cell population.
Fig. 3.
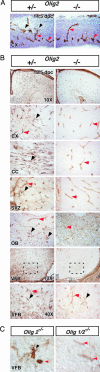
Olig genes are required for NG2 cell development in the brain. (A) IHC for NG2 in 14.5 dpc mouse brain demonstrates the normal appearance of multiprocessed NG2 cells in Olig2+/− mice (black arrowheads) and their absence in _Olig2_−/− mice. NG2 staining in vessel pericytes is unaffected (red arrowheads). (B) IHC at 18.5 dpc demonstrates complete loss of parenchymal NG2 cells (black arrowheads) in the dorsal neocortex (cx), corpus callosum (cc), subventricular zone (svz), and olfactory bulb (ob) of _Olig2_−/− mice. Scattered surviving cells were detected in the ventral forebrain (vfb; boxed regions shown at higher power below). (C) The rare surviving NG2 cells in _Olig2_−/− ventral forebrain had abnormal morphology (black arrowheads). However, analysis of ventral forebrain in Olig1/_2_−/− mice showed complete ablation of normal and abnormal appearing NG2 cells even within the ventral forebrain.
Fig. 4.
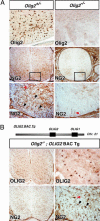
Olig2 function is necessary and sufficient for NG2 cell development in the spinal cord. (A) IHC staining for Olig2 and NG2 in 18.5 dpc Olig2+/− and _Olig2_−/− mouse cervical spinal cord. No cells with NG2 morphology were detected in the absence of Olig2 expression and function. (B) Diagram of 116-kB human BAC clone 2401C4 containing OLIG1 and OLIG2 loci. Crossing of a transgenic mouse line containing this BAC (OLIG2-BAC-Tg) into the _Olig2_−/− mouse line rescues appropriate expression of OLIG2 and promotes OLP development at 18.5 dpc mouse in the spinal cord (30). Concomitant with OLP rescue, NG2 cells with complex morphology were identified (black arrowhead).
Similar articles
- Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum.
Chung SH, Guo F, Jiang P, Pleasure DE, Deng W. Chung SH, et al. Cell Death Dis. 2013 Mar 14;4(3):e546. doi: 10.1038/cddis.2013.74. Cell Death Dis. 2013. PMID: 23492777 Free PMC article. - NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells.
Dromard C, Bartolami S, Deleyrolle L, Takebayashi H, Ripoll C, Simonneau L, Prome S, Puech S, Tran VB, Duperray C, Valmier J, Privat A, Hugnot JP. Dromard C, et al. Stem Cells. 2007 Feb;25(2):340-53. doi: 10.1634/stemcells.2005-0556. Epub 2006 Oct 19. Stem Cells. 2007. PMID: 17053213 - Olig gene function in CNS development and disease.
Ligon KL, Fancy SP, Franklin RJ, Rowitch DH. Ligon KL, et al. Glia. 2006 Jul;54(1):1-10. doi: 10.1002/glia.20273. Glia. 2006. PMID: 16652341 Review. - The Effects of the Olig Family on the Regulation of Spinal Cord Development and Regeneration.
Liu Y, Long ZY, Yang C. Liu Y, et al. Neurochem Res. 2021 Nov;46(11):2776-2782. doi: 10.1007/s11064-021-03383-1. Epub 2021 Jul 6. Neurochem Res. 2021. PMID: 34228233 Review.
Cited by
- Early proliferation does not prevent the loss of oligodendrocyte progenitor cells during the chronic phase of secondary degeneration in a CNS white matter tract.
Payne SC, Bartlett CA, Savigni DL, Harvey AR, Dunlop SA, Fitzgerald M. Payne SC, et al. PLoS One. 2013 Jun 11;8(6):e65710. doi: 10.1371/journal.pone.0065710. Print 2013. PLoS One. 2013. PMID: 23776532 Free PMC article. - Synapses on NG2-expressing progenitors in the brain: multiple functions?
Gallo V, Mangin JM, Kukley M, Dietrich D. Gallo V, et al. J Physiol. 2008 Aug 15;586(16):3767-81. doi: 10.1113/jphysiol.2008.158436. Epub 2008 Jul 17. J Physiol. 2008. PMID: 18635642 Free PMC article. Review. - Origins and clinical implications of the brain tumor stem cell hypothesis.
Zaidi HA, Kosztowski T, DiMeco F, Quiñones-Hinojosa A. Zaidi HA, et al. J Neurooncol. 2009 May;93(1):49-60. doi: 10.1007/s11060-009-9856-x. Epub 2009 May 9. J Neurooncol. 2009. PMID: 19430882 Free PMC article. Review. - NG2 cells: Properties, progeny and origin.
Trotter J, Karram K, Nishiyama A. Trotter J, et al. Brain Res Rev. 2010 May;63(1-2):72-82. doi: 10.1016/j.brainresrev.2009.12.006. Epub 2010 Jan 4. Brain Res Rev. 2010. PMID: 20043946 Free PMC article. Review. - Roles of NG2 glial cells in diseases of the central nervous system.
Xu JP, Zhao J, Li S. Xu JP, et al. Neurosci Bull. 2011 Dec;27(6):413-21. doi: 10.1007/s12264-011-1838-2. Neurosci Bull. 2011. PMID: 22108818 Free PMC article. Review.
References
- Nishiyama A., Lin X. H., Giese N., Heldin C. H., Stallcup W. B. J. Neurosci. Res. 1996;43:299–314. - PubMed
- Stegmuller J., Schneider S., Hellwig A., Garwood J., Trotter J. J. Neurocytol. 2002;31:497–505. - PubMed
- Greenwood K., Butt A. M. Mol. Cell. Neurosci. 2003;23:544–558. - PubMed
- Dawson M. R., Polito A., Levine J. M., Reynolds R. Mol. Cell. Neurosci. 2003;24:476–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 NS047213/NS/NINDS NIH HHS/United States
- R01 NS040511/NS/NINDS NIH HHS/United States
- R01NS40511/NS/NINDS NIH HHS/United States
- K08NS047213/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous