A high-resolution solid-state NMR approach for the structural studies of bicelles - PubMed (original) (raw)
A high-resolution solid-state NMR approach for the structural studies of bicelles
Sergey Dvinskikh et al. J Am Chem Soc. 2006.
Abstract
Bicelles are increasingly being used as membrane mimicking systems in NMR experiments to investigate the structure of membrane proteins. In this study, we demonstrate the effectiveness of a 2D solid-state NMR approach that can be used to measure the structural constraints, such as heteronuclear dipolar couplings between 1H, 13C, and 31P nuclei, in bicelles without the need for isotopic enrichment. This method does not require a high radio frequency power unlike the presently used rotating-frame separated-local-field (SLF) techniques, such as PISEMA. In addition, multiple dipolar couplings can be measured accurately, and the presence of a strong dipolar coupling does not suppress the weak couplings. High-resolution spectra obtained from magnetically aligned DMPC:DHPC bicelles even in the presence of peptides suggest that this approach will be useful in understanding lipid-protein interactions that play a vital role in shaping up the function of membrane proteins.
Figures
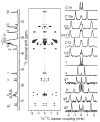
Figure 1
13C chemical shift spectrum (left), 2D spectrum (middle) that correlates the 13C chemical shift (vertical dimension) and 1H-13C dipolar coupling (horizontal dimension) and 1H-13C dipolar coupling slices (right) of 3.5:1 DMPC:DHPC bicelles obtained from a Varian/Chemagnetics 400 MHz solid-state NMR spectrometer. The pulse sequence given in the Supporting Information was used. 64 scans were accumulated for each of 200 points in _t_1 dimensions with the increment time of 384 _μ_s and recycle delay of 5s. 1H rf field strength of 31 kHz were used during _t_1 evolution. Contact time for CP transfer was set to 3.0 ms.
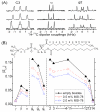
Figure 2
(A) 1H-13C dipolar coupling extracted from 2D spectra from 3.5:1 DMPC:DHPC bicelles with 0 (bottom), 0.5 (middle), and 2 (top) mole % MSI-78. (B) The order parameter profile for DMPC determined from the dipolar coupling values.
Figure 3
(A) A 2D correlation of 13C chemical shift and and 13C-31P dipolar coupling (y-axis) with the 1H chemical shift and 1H-31P dipolar coupling (x-axis) of 3.5:1 DMPC:DHPC bicelles (left). The 1H-31P dipolar split peaks of α, β, g2 and g3 are connected by a green line. (B) The measured 1H-31P (blue) and 13C-31P (red) dipolar couplings in DMPC lipid molecule are highlighted.
Similar articles
- Sensitivity and resolution enhancement of oriented solid-state NMR: application to membrane proteins.
Gopinath T, Mote KR, Veglia G. Gopinath T, et al. Prog Nucl Magn Reson Spectrosc. 2013 Nov;75:50-68. doi: 10.1016/j.pnmrs.2013.07.004. Epub 2013 Aug 12. Prog Nucl Magn Reson Spectrosc. 2013. PMID: 24160761 Free PMC article. Review. - High-resolution 2D NMR spectroscopy of bicelles to measure the membrane interaction of ligands.
Dvinskikh SV, Dürr UH, Yamamoto K, Ramamoorthy A. Dvinskikh SV, et al. J Am Chem Soc. 2007 Jan 31;129(4):794-802. doi: 10.1021/ja065536k. J Am Chem Soc. 2007. PMID: 17243815 Free PMC article. - Lipid concentration and molar ratio boundaries for the use of isotropic bicelles.
Beaugrand M, Arnold AA, Hénin J, Warschawski DE, Williamson PT, Marcotte I. Beaugrand M, et al. Langmuir. 2014 Jun 3;30(21):6162-70. doi: 10.1021/la5004353. Epub 2014 May 19. Langmuir. 2014. PMID: 24797658 Free PMC article. - Proton-evolved local-field solid-state NMR studies of cytochrome b5 embedded in bicelles, revealing both structural and dynamical information.
Soong R, Smith PE, Xu J, Yamamoto K, Im SC, Waskell L, Ramamoorthy A. Soong R, et al. J Am Chem Soc. 2010 Apr 28;132(16):5779-88. doi: 10.1021/ja910807e. J Am Chem Soc. 2010. PMID: 20334357 Free PMC article. - Solid-state NMR structure determination.
Drechsler A, Separovic F. Drechsler A, et al. IUBMB Life. 2003 Sep;55(9):515-23. doi: 10.1080/15216540310001622740. IUBMB Life. 2003. PMID: 14658757 Review.
Cited by
- Sensitivity and resolution enhancement of oriented solid-state NMR: application to membrane proteins.
Gopinath T, Mote KR, Veglia G. Gopinath T, et al. Prog Nucl Magn Reson Spectrosc. 2013 Nov;75:50-68. doi: 10.1016/j.pnmrs.2013.07.004. Epub 2013 Aug 12. Prog Nucl Magn Reson Spectrosc. 2013. PMID: 24160761 Free PMC article. Review. - Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin.
Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. Barry J, et al. J Am Chem Soc. 2009 Apr 1;131(12):4490-8. doi: 10.1021/ja809217u. J Am Chem Soc. 2009. PMID: 19256547 Free PMC article. - Investigating the Mechanisms of AquaporinZ Reconstitution through Polymeric Vesicle Composition for a Biomimetic Membrane.
Zhou D, Zhou H, Zhou S, Tong YW. Zhou D, et al. Polymers (Basel). 2020 Aug 28;12(9):1944. doi: 10.3390/polym12091944. Polymers (Basel). 2020. PMID: 32872107 Free PMC article. - Multiscale simulation of microbe structure and dynamics.
Joshi H, Singharoy A, Sereda YV, Cheluvaraja SC, Ortoleva PJ. Joshi H, et al. Prog Biophys Mol Biol. 2011 Oct;107(1):200-17. doi: 10.1016/j.pbiomolbio.2011.07.006. Epub 2011 Jul 23. Prog Biophys Mol Biol. 2011. PMID: 21802438 Free PMC article. - The magic of bicelles lights up membrane protein structure.
Dürr UH, Gildenberg M, Ramamoorthy A. Dürr UH, et al. Chem Rev. 2012 Nov 14;112(11):6054-74. doi: 10.1021/cr300061w. Epub 2012 Aug 24. Chem Rev. 2012. PMID: 22920148 Free PMC article. Review. No abstract available.
References
- Sanders CR, Hare BJ, Howard KP, Prestegard JH. Prog. Nucl. Magn. Reson. Spectrosc. 1994;26:421–444.
- Sanders CR, Prosser RS. Structure. 1998;6:1227–1234. - PubMed
- Marcotte I, Auger M. Conc. Magn. Reson. A. 2005;24A:17–37.
- Lorigan GA. In: NMR Spectroscopy of Biological Solids. Ramamoorthy A, editor. Taylor & Francis; New York: 2006. Chapter 10.
- Caravatti P, Bodenhausen G, Ernst RR. Chem. Phys. Lett. 1982;89:363–367.
- Nakai T, Terao T. Magn. Reson. Chem. 1992;30:42–44.
- Schmidt-Rohr K, Nanz D, Emsley L, Pines A. J.Phys.Chem. 1994;98:6668–6670.
- Nevzorov AA, Opella SJ. J. Magn. Reson. 2003;164:182–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources