Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1 - PubMed (original) (raw)
Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1
Johanna C Gandy et al. FEBS Lett. 2006.
Abstract
The Ser/Thr kinase Akt1 is activated by growth factors subsequent to its phosphorylation on Thr308 and Ser473. In the present study, Akt1 was found to be constitutively modified with O-GlcNAc. Treatment of SH-SY5Y cells with O(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc), which inhibits the enzymatic removal of O-GlcNAc from proteins, increased cytosolic O-GlcNAc-Akt1 levels. Treatment of cells with insulin-like growth factor-1 (IGF-1) also increased O-GlcNAc-Akt1 levels and increased Akt1 phosphorylation. PUGNAc treatment did not attenuate IGF-1 induced Akt1 phosphorylation. These results indicate that Akt1 can be simultaneously modified with O-GlcNAc and phosphorylated. However, PUGNAc induced the nuclear accumulation of Akt1 suggesting that the O-GlcNAc-modification on Akt1 may play a role in Akt1 nuclear localization.
Figures
Fig. 1
Akt1 is modified with O-GlcNAc. (A) Cytosolic extracts from SH-SY5Y cells were incubated with either unconjugated agarose beads or WGA-conjugated agarose beads. The proteins bound to the unconjugated agarose beads (beads) and WGA-conjugated agarose beads (WGA) were eluted by boiling, and together with the unbound proteins (UB) were immunoblotted with Akt1 antibody. (B) SH-SY5Y cells were cultured in glucose-free media for 24 h, then treated with 20 mM glucose for 1 h. Cytosolic extracts were analyzed for O-GlcNAc-modification of Akt1 using WGA-conjugated agarose beads and cytosolic extracts were immunoblotted for total levels of Akt1. (C) Cytosolic extracts (100 μg of protein) were incubated with 20 units of PNGase F for 20 h in a 37 °C waterbath, and then incubated with WGA-conjugated beads. For β-_N_-acetyl-hexosaminidase (β-hex) treatments, cytosolic extracts were incubated with WGA-conjugated beads as described above, then incubated with 10 units of β-hex for 2 h in a 37 °C waterbath. The eluted proteins were immunoblotted for Akt1. An aliquot from samples without the glycosidases were immunoblotted for Akt1 to verify that proteolysis of Akt1 had not occurred under these conditions. To confirm that β-hex did not affect Akt1 phosphorylation, Akt1 was immunoprecipitated from 100 μg of cytosolic extracts from cells treated with and without IGF-1 (50 ng/ml, 30 min), then incubated in the absence and presence of β-hex, and then immunoblotted with pSer473Akt antibody. The blot was stripped and then reblotted with Akt1 antibody. (D) Cells were treated with 50 μM PUGNAc for 45 min and cytosolic extracts were analyzed for O-GlcNAc-modification of Akt1 using WGA-conjugated beads. Protein complexes were disrupted by the addition of 0.3% SDS during the incubation of the cytosolic extracts with the WGA-conjugated agarose beads. The eluted proteins were immunoblotted for Akt1.
Fig. 2
PUGNAc and IGF-1 increase cytosolic O-GlcNAc levels and O-GlcNAc-modification of Akt1. (A) SH-SY5Y cells were time-dependently treated with 50 μMPUGNAc and 50 ng/ml IGF-1 for the indicated times. Cytosolic extracts were immunoblotted for O-GlcNAc with RL2 antibody. (B) Cytosolic extracts from PUGNAc and IGF-1 treated cells were incubated with WGA-conjugated beads for analysis of O-GlcNAc-modification of Akt1. O-GlcNAc modified Akt1 bands were quantitated by scanning densitometry. Means ± S.E., n = three experiments; *P < 0.05 (ANOVA) compared to values from untreated cells. Cytosolic extracts were immunoblotted with pThr308Akt1, pSer473Akt, and Akt1 antibodies. (C) For measurement of Akt1 activity, cells were time-dependently treated with 50 μM PUGNAc or treated with IGF-1 (50 ng/ml, 30 min). The cytosolic extracts were immunoprecipitated with Akt1 antibody and incubated with GSK3 fusion protein substrate. The reaction mixture was immunoblotted with a GSK3 phospho-specific antibody. To determine efficiency of immunoprecipitation the blot was stripped and reblotted with Akt1 antibody.
Fig. 3
Akt1 can be simultaneously modified with O-GlcNAc and phosphorylated on Thr308 and Ser473. (A) SH-SY5Y cells were stimulated with 50 ng/ml IGF-1 for 30 min. Cytosolic extracts were incubated with WGA-conjugated agarose beads, and the eluted proteins were immunoblotted with pThr308Akt1, pSer473Akt1, and Akt1 antibodies. (B) HEK293 cells were transiently transfected with wild-type HA-tagged Akt1 or an HA-tagged Akt1 mutant in which the Thr308 and Ser473 were dually mutated to Asp (DDAkt). Cytosolic extracts were incubated with WGA-conjugated agarose beads and eluted proteins were immunoblotted with HA antibody. (C) SH-SY5Y cells were treated with 50 μM PUGNAc 30 min prior to treatment with IGF-1 (50 ng/ml, 30 min). Cytosolic extracts were immunoblotted with pThr308Akt1, pSer473Akt1, Akt1, pSer9GSK3β, and GSK3β antibodies. (D) Cells were treated with 20 μM LY294002 30 min prior to treatment with 50 ng/ml IGF-1 for 30 min or 50 μM PUGNAc for 30 min. Cytosolic extracts were incubated with WGA-conjugated beads for analysis of O-GlcNAc-modification of Akt1, and cytosolic extracts from IGF-1 and LY294002-treated cells were also immunoblotted for pThr308Akt and Akt1.
Fig. 4
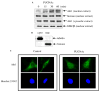
PUGNAc induces nuclear accumulation of Akt1. (A) SH-SY5Y cells were treated with 50 μM PUGNAc for the indicated times. Nuclear and cytosolic extracts were immunoblotted with Akt1 antibody and nuclear extracts with histone antibody and GSK3β antibody. (B) Complete separation of the cytosolic and nuclear fractions was confirmed by immunoblotting the cytosolic and nuclear extracts with tubulin and histone antibodies. (C) Control and PUGNAc-treated (50 μM, 30 min) cells were immunofluorescently labeled with Akt1-fluorescein isothiocyanateconjugated antibody, upper panels. Nuclei were stained with Hoechst 33342, lower panels.
Similar articles
- Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes.
Vosseller K, Wells L, Lane MD, Hart GW. Vosseller K, et al. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5313-8. doi: 10.1073/pnas.072072399. Proc Natl Acad Sci U S A. 2002. PMID: 11959983 Free PMC article. - Increasing O-GlcNAc levels: An overview of small-molecule inhibitors of O-GlcNAcase.
Macauley MS, Vocadlo DJ. Macauley MS, et al. Biochim Biophys Acta. 2010 Feb;1800(2):107-21. doi: 10.1016/j.bbagen.2009.07.028. Epub 2009 Aug 4. Biochim Biophys Acta. 2010. PMID: 19664691 Review. - O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar.
Wells L, Hart GW. Wells L, et al. FEBS Lett. 2003 Jul 3;546(1):154-8. doi: 10.1016/s0014-5793(03)00641-0. FEBS Lett. 2003. PMID: 12829252 Review.
Cited by
- O-GlcNAc signaling: a metabolic link between diabetes and cancer?
Slawson C, Copeland RJ, Hart GW. Slawson C, et al. Trends Biochem Sci. 2010 Oct;35(10):547-55. doi: 10.1016/j.tibs.2010.04.005. Epub 2010 May 11. Trends Biochem Sci. 2010. PMID: 20466550 Free PMC article. Review. - The O-GlcNAc Modification on Kinases.
Schwein PA, Woo CM. Schwein PA, et al. ACS Chem Biol. 2020 Mar 20;15(3):602-617. doi: 10.1021/acschembio.9b01015. Epub 2020 Mar 10. ACS Chem Biol. 2020. PMID: 32155042 Free PMC article. - Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-Linked beta-N-acetylglucosamine in 3T3-L1 adipocytes.
Whelan SA, Dias WB, Thiruneelakantapillai L, Lane MD, Hart GW. Whelan SA, et al. J Biol Chem. 2010 Feb 19;285(8):5204-11. doi: 10.1074/jbc.M109.077818. Epub 2009 Dec 17. J Biol Chem. 2010. PMID: 20018868 Free PMC article. - Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose.
Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH. Hu Y, et al. J Biol Chem. 2009 Jan 2;284(1):547-555. doi: 10.1074/jbc.M808518200. Epub 2008 Nov 12. J Biol Chem. 2009. PMID: 19004814 Free PMC article. - Altered expression of O-GlcNAc-modified proteins in a mouse model whose glycemic status is controlled by a low carbohydrate ketogenic diet.
Okuda T, Fukui A, Morita N. Okuda T, et al. Glycoconj J. 2013 Nov;30(8):781-9. doi: 10.1007/s10719-013-9482-x. Epub 2013 Jun 21. Glycoconj J. 2013. PMID: 23793825
References
- Brazil DP, Hemmings BA. Ten years of protein kinase B signaling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. - PubMed
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. - PubMed
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous