The biological actions of dehydroepiandrosterone involves multiple receptors - PubMed (original) (raw)
Review
The biological actions of dehydroepiandrosterone involves multiple receptors
Stephanie J Webb et al. Drug Metab Rev. 2006.
Abstract
Dehydroepiandrosterone has been thought to have physiological functions other than as an androgen precursor. The previous studies performed have demonstrated a number of biological effects in rodents, such as amelioration of disease in diabetic, chemical carcinogenesis, and obesity models. To date, activation of the peroxisome proliferators activated receptor alpha, pregnane X receptor, and estrogen receptor by DHEA and its metabolites have been demonstrated. Several membrane-associated receptors have also been elucidated leading to additional mechanisms by which DHEA may exert its biological effects. This review will provide an overview of the receptor multiplicity involved in the biological activity of this sterol.
Figures
Figure 1
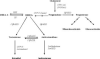
Metabolic pathway for DHEA synthesis and utilization. The biosynthetic pathway from cholesterol to various sterol metabolites. The enzymes involved are: CYP11A1 (P450scc or cholesterol side-chain cleavage P450 enzyme); CYP17 (lyase or steroid 17 alpha-hydroxylase/17,20 lyase); CYP 19 (aromatase); 3α or β-HSD, 3α or β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; 17β-KSR, 17β-ketosteroid reductase; HSS, hydroxysteroid sulfotransferase; SS, sterol sulfatase.
Figure 2
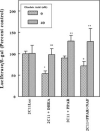
DHEA and its metabolites do not activate PPARα in cell-based assays. HepG2 cells were transfected with a luciferase construct containing the peroxisome proliferators responsive element from murine fatty acyl CoA oxidase, an expression plasmid for murine PPARα, and an expression plasmid for β-galactosidase. The transfected cells were treated with 50 μM concentrations of nafenopin, a known peroxisome proliferating chemical, DHEA sulfate, DHEA, or a series of its oxidized metabolites (7-keto-DHEA, 7α-hydroxy-DHEA, 7β-hydroxy-DHEA, ADIOL, or ADIONE.) The cells were harvested 24 hours later and assayed for β-galactosidase and luciferase activity. The values are the average of three samples ± standard deviation.
Figure 3
DHEA and its metabolites induce P4504A1 message in primary cultures of rat hepatocytes. Rat hepatocytes were isolated as described previously (Prough et al., 1995) and treated with 50 μM concentrations of nafenopin, DHEA sulfate, DHEA, or a series of its oxidized metabolites (ADIOL, DHEA acetate, 7α-hydroxy-DHEA, 7-oxo-DHEA, 16α-hydroxy-DHEA, 7-oxo-ADIOL, or 7-oxo-ADIOL acetate). The mRNA was isolated and analyzed by Northern analysis; the density of each band was measured using a densitometer. Inset: The concentration-response obtained for increasing concentrations of 7-oxo-DHEA. The EC50 value of 7.2 μM was observed for 7-oxo-DHEA. The values are the average of three samples ± standard deviation.
Figure 4
DHEA activates estrogen receptor α and β in HepG2 cells. HepG2 cells were transfected with a luciferase construct containing a canonical estrogen responsive element, expression plasmids for either human ERα or ERβ, and an expression plasmid for β-galactosidase. The transfected cells were treated with 50 μM concentrations of DHEA or DHEA sulfate. For an estrogen standard, 17β-estradiol was added at a concentration of 50 nM. The cells were harvested 24 hours later and assayed for β-galactosidase and luciferase activity. The values are the average of three samples ± standard deviation.
Figure 5
Effect of okadaic acid on activation of a P4502C11 reporter construct by DHEA and Nafenopin. HepG2 cells were transfected with a luciferase construct containing the 5′-flanking region of rat P4502C11 gene, expression plasmids for murine PPARα, and an expression plasmid for β-galactosidase. The transfected cells were treated with either 50 μM DHEA or nafenopin. The cells were harvested 24 hours later and assayed for β-galactosidase and luciferase activity. The values are the average of three samples ± standard deviation.
Figure 6
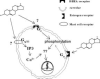
Membrane-associated receptors for DHEA. The scheme shows several mechanisms of membrane-associated receptor activation by DHEA. One involves a caveole-associated receptor for DHEA acting through a Giα receptor system (Widstrom and Dillon, 2004), one involves membrane-associate estrogen receptor α or β, and one involves a Gq/11protein-coupled membrane receptor found in mast cells (Ueda et al., 2001).
Figure 7
Expression of 11β-hydroxysteroid dehydrogenase is regulated by peroxisome proliferators. HepG2 cells were transfected with a luciferase construct containing the 5′-flanking region of 11β-HSD, expression plasmids for murine PPARα, and an expression plasmid for β-galactosidase. In addition, an expression plasmid for C/EBP was transfected in certain experiments. The transfected cells were treated with 50 μM nafenopin. The cells were harvested 24 hours later and assayed for β-galactosidase and luciferase activity. The values are the average of three samples ± standard deviation.
Figure 8
DHEA and the peroxisome proliferators nafenopin induce expression of IGFBP-1 in rat liver. Male Sprague-Dawley rats were maintained on an ad libitum diet of AIN76A chow and administered either DHEA per os (0.45% DHEA in AIN76A chow) for 5 days, or nafenopin by daily i.p. injection for four days (80 mg/kg). First-strand reverse transcription was performed on 1 μg of total hepatic RNA and used for measuring changes in IGFBP-1 expression by quantitative RT-PCR using gene-specific primers. Values represent the average and standard deviation of the absolute mRNA quantities normalized to 18S rRNA from three independent animals per treatment group.
Similar articles
- Induction of CYP3A expression by dehydroepiandrosterone: involvement of the pregnane X receptor.
Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. Ripp SL, et al. Drug Metab Dispos. 2002 May;30(5):570-5. doi: 10.1124/dmd.30.5.570. Drug Metab Dispos. 2002. PMID: 11950789 - Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate.
Peters JM, Zhou YC, Ram PA, Lee SS, Gonzalez FJ, Waxman DJ. Peters JM, et al. Mol Pharmacol. 1996 Jul;50(1):67-74. Mol Pharmacol. 1996. PMID: 8700121 - Dehydroepiandrosterone Research: Past, Current, and Future.
Klinge CM, Clark BJ, Prough RA. Klinge CM, et al. Vitam Horm. 2018;108:1-28. doi: 10.1016/bs.vh.2018.02.002. Epub 2018 Mar 16. Vitam Horm. 2018. PMID: 30029723 Review. - The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways.
Kliewer SA, Lehmann JM, Milburn MV, Willson TM. Kliewer SA, et al. Recent Prog Horm Res. 1999;54:345-67; discussion 367-8. Recent Prog Horm Res. 1999. PMID: 10548883 Review. - Novel mechanisms for DHEA action.
Prough RA, Clark BJ, Klinge CM. Prough RA, et al. J Mol Endocrinol. 2016 Apr;56(3):R139-55. doi: 10.1530/JME-16-0013. Epub 2016 Feb 23. J Mol Endocrinol. 2016. PMID: 26908835 Review.
Cited by
- Examining the Effects of Nutrient Supplementation on Metabolic Pathways via Mitochondrial Ferredoxin in Aging Ovaries.
Wu CC, Li CJ, Lin LT, Wen ZH, Cheng JT, Tsui KH. Wu CC, et al. Nutrients. 2024 May 13;16(10):1470. doi: 10.3390/nu16101470. Nutrients. 2024. PMID: 38794708 Free PMC article. - The Effects of the Steroids 5-Androstenediol and Dehydroepiandrosterone and Their Synthetic Derivatives on the Viability of K562, HeLa, and Wi-38 Cells and the Luminol-Stimulated Chemiluminescence of Peripheral Blood Mononuclear Cells from Healthy Volunteers.
Sokolov MN, Rozhkov VV, Uspenskaya ME, Ulchenko DN, Shmygarev VI, Trukhan VM, Churakov AV, Shimanovsky NL, Fedotcheva TA. Sokolov MN, et al. Biomolecules. 2024 Mar 19;14(3):373. doi: 10.3390/biom14030373. Biomolecules. 2024. PMID: 38540791 Free PMC article. - High Pretreatment DHEA Is Associated with Inferior Immunotherapy Response in Metastatic Non-Small Cell Lung Cancer.
Zhang Y, Darville L, Hogue S, Hallanger Johnson JE, Rose T, Kim Y, Bailey A, Gray JE, Robinson LA. Zhang Y, et al. Cancers (Basel). 2024 Mar 14;16(6):1152. doi: 10.3390/cancers16061152. Cancers (Basel). 2024. PMID: 38539486 Free PMC article. - Effects of low doses of the novel dehydroepiandrosterone (DHEA) derivative BNN27 in rat models of anxiety.
Fragkiadaki E, Katsanou L, Vartzoka F, Gravanis A, Pitsikas N. Fragkiadaki E, et al. Psychopharmacology (Berl). 2024 Feb;241(2):341-350. doi: 10.1007/s00213-023-06490-9. Epub 2023 Nov 2. Psychopharmacology (Berl). 2024. PMID: 37917180 Free PMC article. - New Possibilities for Hormonal Vaginal Treatment in Menopausal Women.
Tomczyk K, Chmaj-Wierzchowska K, Wszołek K, Wilczak M. Tomczyk K, et al. J Clin Med. 2023 Jul 18;12(14):4740. doi: 10.3390/jcm12144740. J Clin Med. 2023. PMID: 37510854 Free PMC article. Review.
References
- Abuissa H, Bel DS, O'Keefe JH., Jr. Strategies to prevent type 2 diabetes. Curr. Med. Res. Opin. 2005;21:1107–1114. - PubMed
- Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol. Metab. 2002;13:288–294. - PubMed
- Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2005;288:E957–E964. - PubMed
- Arad Y, Badimon JJ, Badimon L, Hembree WC, Ginsberg HN. Dehydroepiandrosterone feeding prevents aortic fatty streak formation and cholesterol accumulation in cholesterol-fed rabbit. Arteriosclerosis. 1989;9:159–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK054774-08/DK/NIDDK NIH HHS/United States
- R01 DK054774-04/DK/NIDDK NIH HHS/United States
- R01 DK054774-02/DK/NIDDK NIH HHS/United States
- R01 DK054774-05/DK/NIDDK NIH HHS/United States
- DK54774/DK/NIDDK NIH HHS/United States
- R01 DK054774-06/DK/NIDDK NIH HHS/United States
- R01 DK054774-01/DK/NIDDK NIH HHS/United States
- R01 DK054774-07/DK/NIDDK NIH HHS/United States
- R01 DK054774-03/DK/NIDDK NIH HHS/United States
- R01 DK054774/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources