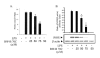Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia - PubMed (original) (raw)
Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia
Manu Jatana et al. J Neuroinflammation. 2006.
Abstract
Background: Stroke is one of the leading causes of death worldwide and a major cause of morbidity and mortality in the United States of America. Brain ischemia-reperfusion (IR) triggers a complex series of biochemical events including inflammation. Leukotrienes derived from 5-lipoxygenase (5-LOX) cause inflammation and are thus involved in the pathobiology of stroke injury.
Methods: To test the neuroprotective efficacy of 5-LOX inhibition in a rat model of focal cerebral IR, ischemic animals were either pre- or post-treated with a potent selective 5-LOX inhibitor, (N- [3-[3-(-fluorophenoxy) phenyl]-1-methyl-2-propenyl]-N-hydroxyurea (BW-B 70C). They were evaluated at 24 h after reperfusion for brain infarction, neurological deficit score, and the expression of 5-LOX. Furthermore, the mechanism and the anti-inflammatory potential of BW-B 70C in the regulation of nuclear factor kappa B (NF-kappaB) and inflammatory inducible nitric oxide synthase (iNOS) were investigated both in vivo and in vitro.
Results and discussion: Both pre- and post-treatment with BW-B 70C reduced infarctions and improved neurological deficit scores. Immunohistochemical study of brain sections showed IR-mediated increased expression of 5-LOX in the neurons and microglia. BW-B 70C down-regulated 5-LOX and inhibited iNOS expression by preventing NF-kappaB activation. Two other structurally different 5-LOX inhibitors were also administered post IR: caffeic acid and 2,3,5-trimethyl-6-[12-hydroxy-5,10-dodecadiynyl]-1,4-benzoquinone (AA-861). As with BW-B 70C, they provided remarkable neuroprotection. Furthermore, in vitro, BW-B 70C inhibited lipopolysaccharide (LPS) mediated nitric oxide production, iNOS induction and NF-kappaB activation in the BV2 microglial cell line. Treating rat primary microglia with BW-B70C confirmed blockage of LPS-mediated translocation of the p65 subunit of NF-kappaB from cytosol to nucleus.
Conclusion: The study demonstrates the neuroprotective potential of 5-LOX inhibition through down-regulation of NF-kappaB in a rat model of experimental stroke.
Figures
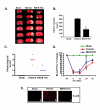
Figure 1
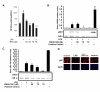
Pretreatment with BW-B 70C protects the brain from infarction and improves neurological score. (A) Photograph showing effect of BW-B 70C on TTC-stained sections, (B) Effect of BW-B 70C on infarct volume (measured in six serial coronal sections arranged from cranial to caudal regions), (C) Effect of BW-B 70C on neurological score and (D) Effect of BW-B 70C on regional cerebral blood flow (CBF). Changes in CBF were not significantly different after ischemia between the untreated (vehicle) and treatment (BW-B 70C) groups. (E) Photomicrograph of expression of 5-LOX (n = 4) at 24 h reperfusion after 20 min MCAO (magnification X200). Data for infarct volume (n = 7) and blood flow (n = 4) are presented as means ± SD. *p < 0.001 vs. vehicle. Data for neurological deficit score (n = 7) are presented as individual data points.
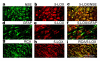
Figure 2
5-LOX is expressed in neurons and microglia/macrophages. Co-localization of expression of (a) NSE, (d) GFAP; (g) RCA; and 5-LOX (b,e,h) at 24 h reperfusion after 20 min MCAO. Yellow fluorescence indicates co-localization of 5-LOX/NSE (c) and 5-LOX/RCA (i). 5-LOX/GFAP (f) showed very few yellow-fluorescent structures. Figures are representative of similar results obtained from three different sections of three different animals in each group (Magnification 400 ×).
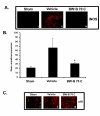
Figure 3
BW-B 70C inhibits expression of iNOS and p65 in vivo. (A) Photomicrographs of immunohistochemistry of rat brain sections at 24 h reperfusion after 20 min MCAO showing remarkable iNOS expression in vehicle-treated (ii) but not in BW-B 70C-treated rats (iii). (B) BW-B 70C inhibited IR-induced iNOS gene expression measured as mRNA levels at 3 h of reperfusion after 20 min MCAO. The results are presented as mean ± SD of normalized expression of target gene with respect to GAPDH mRNA from three sets of animals. (C) Treatment with BW-B 70C prevented the nuclear translocation of p65 subunit of NF-κB (i-iii); the vehicle-treated animals showed nuclear translocation (ii) and treatment with BW-B 70C reversed this (iii) at 24 h reperfusion after 20 min MCAO. Figures are representative of similar results obtained from three groups of animals. A (i-iii) magnification 100 X; C (i-iii) magnification 200 X. *p < 0.01 vs. vehicle (n = 3).
Figure 4
BW-B 70C inhibits LPS-mediated iNOS expression in BV2 cells. (A) BV2 Cells were pretreated for 30 min with different concentrations of BW-B 70C followed by LPS (1 μg/ml) treatment. After 24 h, NO as nitrite was quantified in supernatant by Griess reagent. Data are presented as means ± SD for 3 different experiments. (B) Cell lysates were processed for western blot analysis of iNOS and β-actin after 24 h of stimulation with LPS (1 μg/ml). Figures are representative of 3 different experiments. *p < 0.001 vs. LPS; **p < 0.001 vs. LPS+25 μM BW-B70C; ***p < 0.001 vs. LPS+25 μM BW-B70C; +p < 0.001vs LPS + 50 μM BW-B 70C.
Figure 5
BW-B 70C inhibits LPS-induced NF-κB activation in microglial BV2 cells and prevents nuclear translocation of p65 in rat primary microglia. (A) Microglial cells (BV2) were transiently co-transfected with 1.5 μg of NF-κB luciferase reporter construct. Cells were pre-treated with BW-B 70C (25–75 μM) for 30 min followed by LPS (1 μg/ml) stimulation for 4 h. Luciferase activity was normalized with respect to β-galactosidase activity. Data are means ± SD of three different experiments. Immunoblot was performed at 1 h post treatment with LPS (1 μg/ml) for p65 (B) and p50 (C) in nuclear extract from primary microglia. A non-specific band (NS) was taken as internal standard. Blots are representative of three different experiments. (D) Immunohistochemcal analysis of rat primary microglia showing nuclear translocation of p65 1 h post LPS treatment. Cells were pretreated with BW-B 70C (75 μM) for 30 min before stimulation with LPS (1 μg/ml). Red fluorescence shows positive reaction for p65 and blue fluorescence showed nuclear staining with DAPI. LPS treatment translocated p65 to the nucleus, and treatment with BW-B 70C reversed it. Figures are representative of 3 experiments. (Magnification 200 X). #p < 0.001 vs. untreated; *p < 0.001 vs. untreated; **p < 0.001 vs. LPS; ***p < 0.001 vs. LPS+BW-B 70C (50 μM).
Similar articles
- Effects of Flower Buds Extract of Tussilago farfara on Focal Cerebral Ischemia in Rats and Inflammatory Response in BV2 Microglia.
Hwang JH, Kumar VR, Kang SY, Jung HW, Park YK. Hwang JH, et al. Chin J Integr Med. 2018 Nov;24(11):844-852. doi: 10.1007/s11655-018-2936-4. Epub 2018 Aug 8. Chin J Integr Med. 2018. PMID: 30090976 - Ruscogenin reduces cerebral ischemic injury via NF-κB-mediated inflammatory pathway in the mouse model of experimental stroke.
Guan T, Liu Q, Qian Y, Yang H, Kong J, Kou J, Yu B. Guan T, et al. Eur J Pharmacol. 2013 Aug 15;714(1-3):303-11. doi: 10.1016/j.ejphar.2013.07.036. Epub 2013 Jul 30. Eur J Pharmacol. 2013. PMID: 23911884 - Trans-Cinnamaldehyde, An Essential Oil in Cinnamon Powder, Ameliorates Cerebral Ischemia-Induced Brain Injury via Inhibition of Neuroinflammation Through Attenuation of iNOS, COX-2 Expression and NFκ-B Signaling Pathway.
Chen YF, Wang YW, Huang WS, Lee MM, Wood WG, Leung YM, Tsai HY. Chen YF, et al. Neuromolecular Med. 2016 Sep;18(3):322-33. doi: 10.1007/s12017-016-8395-9. Epub 2016 Apr 18. Neuromolecular Med. 2016. PMID: 27087648 - Isobutyrylshikonin inhibits lipopolysaccharide-induced nitric oxide and prostaglandin E2 production in BV2 microglial cells by suppressing the PI3K/Akt-mediated nuclear transcription factor-κB pathway.
Jayasooriya RG, Lee KT, Kang CH, Dilshara MG, Lee HJ, Choi YH, Choi IW, Kim GY. Jayasooriya RG, et al. Nutr Res. 2014 Dec;34(12):1111-9. doi: 10.1016/j.nutres.2014.10.002. Epub 2014 Oct 7. Nutr Res. 2014. PMID: 25454762
Cited by
- Promoting endothelial function by S-nitrosoglutathione through the HIF-1α/VEGF pathway stimulates neurorepair and functional recovery following experimental stroke in rats.
Khan M, Dhammu TS, Matsuda F, Baarine M, Dhindsa TS, Singh I, Singh AK. Khan M, et al. Drug Des Devel Ther. 2015 Apr 17;9:2233-47. doi: 10.2147/DDDT.S77115. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25945035 Free PMC article. - Cytochrome P450 derived epoxidized fatty acids as a therapeutic tool against neuroinflammatory diseases.
Atone J, Wagner K, Hashimoto K, Hammock BD. Atone J, et al. Prostaglandins Other Lipid Mediat. 2020 Apr;147:106385. doi: 10.1016/j.prostaglandins.2019.106385. Epub 2019 Nov 5. Prostaglandins Other Lipid Mediat. 2020. PMID: 31698143 Free PMC article. Review. - Inhibition of lipoxygenases and cyclooxygenases by linoleyl hydroxamic acid: comparative in vitro studies.
Butovich IA, Lukyanova SM. Butovich IA, et al. J Lipid Res. 2008 Jun;49(6):1284-94. doi: 10.1194/jlr.M700602-JLR200. Epub 2008 Feb 27. J Lipid Res. 2008. PMID: 18305312 Free PMC article. - 5-Lypoxygenase products are involved in renal tubulointerstitial injury induced by albumin overload in proximal tubules in mice.
Landgraf SS, Silva LS, Peruchetti DB, Sirtoli GM, Moraes-Santos F, Portella VG, Silva-Filho JL, Pinheiro CS, Abreu TP, Takiya CM, Benjamin CF, Pinheiro AA, Canetti C, Caruso-Neves C. Landgraf SS, et al. PLoS One. 2014 Oct 10;9(10):e107549. doi: 10.1371/journal.pone.0107549. eCollection 2014. PLoS One. 2014. PMID: 25302946 Free PMC article. - 5-Lipoxygenase inhibitor zileuton inhibits neuronal apoptosis following focal cerebral ischemia.
Shi SS, Yang WZ, Tu XK, Wang CH, Chen CM, Chen Y. Shi SS, et al. Inflammation. 2013 Dec;36(6):1209-17. doi: 10.1007/s10753-013-9657-4. Inflammation. 2013. PMID: 23695166
References
- Iadecola C, Zhang F, Xu S, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following cerebral ischemia. J Cereb Blood Flow Metab. 1995;15:378–384. - PubMed
- Willmot M, Gibson C, Gray L, Murphy S, Bath P. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: A systematic review. Free Radical Biology and Medicine. 2005;39:412–425. doi: 10.1016/j.freeradbiomed.2005.03.028. - DOI - PubMed
Grants and funding
- R01 NS022576/NS/NINDS NIH HHS/United States
- R01 NS034741/NS/NINDS NIH HHS/United States
- R37 NS022576/NS/NINDS NIH HHS/United States
- R01 NS040810/NS/NINDS NIH HHS/United States
- C06 RR018823/RR/NCRR NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- R01 NS040144/NS/NINDS NIH HHS/United States
- R01 NS037766/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials