Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear - PubMed (original) (raw)
Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear
Sarah Pauley et al. Dev Dyn. 2006 Sep.
Abstract
The forkhead genes are involved in patterning, morphogenesis, cell fate determination, and proliferation. Several Fox genes (Foxi1, Foxg1) are expressed in the developing otocyst of both zebrafish and mammals. We show that Foxg1 is expressed in most cell types of the inner ear of the adult mouse and that Foxg1 mutants have both morphological and histological defects in the inner ear. These mice have a shortened cochlea with multiple rows of hair cells and supporting cells. Additionally, they demonstrate striking abnormalities in cochlear and vestibular innervation, including loss of all crista neurons and numerous fibers that overshoot the organ of Corti. Closer examination shows that some anterior crista fibers exist in late embryos. Tracing these fibers shows that they do not project to the brain but, instead, to the cochlea. Finally, these mice completely lack a horizontal crista, although a horizontal canal forms but comes off the anterior ampulla. Anterior and posterior cristae, ampullae, and canals are reduced to varying degrees, particularly in combination with Fgf10 heterozygosity. Compounding Fgf10 heterozygotic effects suggest an additive effect of Fgf10 on Foxg1, possibly mediated through bone morphogenetic protein regulation. We show that sensory epithelia formation and canal development are linked in the anterior and posterior canal systems. Much of the Foxg1 phenotype can be explained by the participation of the protein binding domain in the delta/notch/hes signaling pathway. Additional Foxg1 effects may be mediated by the forkhead DNA binding domain.
Copyright 2006 Wiley-Liss, Inc.
Figures
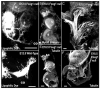
Fig. 1
Adult Foxg1 expression analysis using LacZ. Strong Foxg1 expression is seen in the cochlea, saccule, utricle, and canal cristae. Weaker Foxg1 expression is seen in the canals, particularly near the ampullae of the cristae; and is strongest at the inner and outer curvatures. In the utricle, an expression gradient can be seen from stronger to weaker expression in the neuronal to abneuronal portions, respectively. A: There is a faint gradient in the cochlea with the base being less stained than the apex. B: The horizontal crista sensory epithelium is outlined to show that the Foxg1 expression is limited to the supporting cells of the sensory epithelia. C: An overview of the cochlea shows very intense expression in the spiral ganglion, the inner spiral sulcus, and the organ of Corti except for the cell-free tunnel of Corti. D,E: Radial sections through the cochlea show intense staining in the Reissner’s membrane, spiral limbus, and inner spiral sulcus (below the tectorial membrane). The inner hair cells are strongly labeled, whereas outer hair cells are faintly labeled. All supporting cells are labeled, including border cells, inner and outer pillar cells, Deiter’s cells, and Hensen’s cells. The outer spiral sulcus, or Claudius’ cells are strongly labeled, but the stria vascularis is strikingly devoid of staining, as is the otic mesenchyme and the basilar membrane. F: The vestibular ganglion shows Foxg1 in all neurons, but the intensity is variable. G: The utricle of the adult shows staining in some hair cells and all supporting cells on the neuronal side with a rapid decrease of expression on the abneuronal side. A,C,D,H: All spiral ganglion neurons demonstrate Foxg1 expression to varying degrees. PC, posterior crista; HC, horizontal crista; AC, anterior crista; U, utricle; S, saccule; B, base; A, apex; SpGl, spiral ganglion; SpSl, spiral limbus; OC, organ of Corti; RM, Reissner’s membrane; SV, stria vascularis; TM, tectorial membrane; SL, spiral limbus; BM, basilar membrane; IHC, inner hair cell; OHC, outer hair cell; BC, border cell; IP, inner pillar; TC, tunnel of Corti; OP, outer pillar; DC, Deiter’s cell; HC, Hensen’s cell; HCs, hair cells; SCs, supporting cells. Scale bars = 100 μm in A–D,F–H, 10 μm in E.
Fig. 2
Morphogenesis of the Foxg1 null inner ear. A,C,E: Foxg1 in heterozygotic animals is expressed in the entire presumptive cochlea, the inferior and superior vestibular ganglia, the spiral ganglion, the geniculate ganglia, utricle, saccule, and all canal cristae. There is weaker expression in the developing anterior and posterior canals (but not the horizontal canal), and in the endolymphatic duct. B,D,F: In the Foxg1 null mutant, the LacZ expression pattern is basically the same as in the wild-type at E11.5. E,F: Whereas the size of the cochlea and ganglia appear to be normal, there is reduced anterior–posterior extension of the canal plates and the posterior plates that would normally fuse to form the posterior canal (E), remains unfused (F). D: The cochlea shows a delay in spiraling. Additionally, the mutant shows LacZ expression in the horizontal canal, whereas there is no Foxg1 expression in the horizontal canal of the Foxg1 heterozygote. C–F: The Foxg1 heterozygotes show a distinct horizontal crista sensory patch (C,E) that is completely absent in Foxg1 null mice (D,F). Note the more extensive reaction in Foxg1 null mice that is likely due to the double expression of LacZ under Foxg1 promoter control. AC, anterior crista; PC, posterior crista; ED, endolymphatic duct; SVG, superior vestibular ganglion; IVG, inferior vestibular ganglion; GG, geniculate ganglion; Coch, cochlea; U, utricle; S, saccule; SpGl, spiral ganglion. Scale bar = 100 μm in all images, anterior is to the left, dorsal is up.
Fig. 3
A–I: Morphological defects of the Foxg1 null at embryonic day (E) 18.5. D,H: Foxg1 heterozygotic animals have fully developed the three canals and the common crus and show Foxg1 expression in all canal cristae, utricle, saccule, and cochlea, much like the adult pattern of expression. A,C: In contrast, Foxg1 mutants, particularly on an Fgf10 heterozygotic background, have a reduced (C) or no posterior crista or posterior canal (A, not reacted for LacZ). A–C,F: All Foxg1 mutants lack a horizontal crista but do have a horizontal canal. A–C: All Foxg1 mutants have a shortened, wide cochlea (A–C) with a reduced spiral ganglion (B) that does not spiral along the cochlea. E,F,H: The anterior crista of Foxg1 mutants is somewhat smaller than that of the wild-type and is asymmetrical around the cruciate eminence. F: Note the absence of a horizontal crista in Foxg1 mutants and that the horizontal canal branches off the same ampulla as the anterior crista. F–I: The posterior crista of Foxg1 null mice is much smaller compared with the posterior crista of the wild-type. Additionally, there is no sign of a cruciate eminence. D,E,G,H,I: Note that the utricle shows less intense staining near the striolar region (D,H) and that the canal cristae have extensive staining in both hair cells and supporting cells (E,G,I). AC, anterior crista; HC, horizontal canal or horizontal crista; CC, common crus; PC, posterior crista; SpGl, spiral ganglion; U, utricle; S, saccule. Scale bars = 100 μm in A-D,F,H; 50 μm in E,G,I.
Fig. 4
Multiple rows of hair cells form in the apex of the Foxg1 mutant at embryonic day (E) 18.5. A: MYO VIIa immunostaining, a marker for hair cells, shows three rows of outer hair cells and one row of inner hair cells along the length of the cochlea in the wild-type at E18.5. B: In contrast, MYO VIIa immunostaining shows multiple rows of outer and inner hair cells near the apex in Foxg1 null mice. C,D: Likewise, in situ hybridization for Atoh1, a marker for differentiating hair cells, in the Foxg1 null cochlea shows that the organ of Corti widens to multiple rows of hair cells near the apex. E,F: The dotted line represents the approximate location of the near radial epoxy sections of a lacZ-reacted Foxg1 mutant cochlea. Up to 15 rows of poorly differentiated hair cells can be identified, partially segregated into outer and inner hair cells. The spiral artery is visible running below the organ of Corti. This artery normally runs below the tunnel of Corti and can be used as a landmark to differentiate between inner and outer hair cells and pillar cells. F: In the Foxg1 null, this landmark is of little use as the artery is tortuous and we are unable to distinguish any of the supporting cells as pillar cells and there appears to be no tunnel of Corti. Reissner’s membrane and Claudius’ cells were prominently stained. One or two rows of Hensen’s cells capped the lateral edge of the organ of Corti. F: The greater epithelial ridge showed less reaction. IHCs, inner hair cells; OHCs, outer hair cells; GER, greater epithelial ridge; CCs, Claudius’ cells; SCs, supporting cells; SPA, spiral artery; RM, Reissner’s membrane. Scale bars = 100 μm in A–D; 10 μm in E,F.
Fig. 5
Multiple rows of hair cells are revealed by scanning electron microscopy. A: At embryonic day (E) 18.5 in the Foxg1 null, the cochlea shows almost one full turn. B–D: Hair cells throughout the cochlea show mature apical specializations including a kinocilium and stereocilia. C: Near the base, the organization of the hair cells is fairly normal. There is an additional row of outer hair cells for a total of four distinct rows, and the normal, single row of inner hair cells. Additionally, the polarity of the hair cells is normal as illustrated by the arrows. D: In the middle of this shortened cochlea, we see many more disorganized rows of hair cells and it is difficult to differentiate between inner and outer hair cells. Furthermore, the polarity of the hair cells, although generally correct, is less consistent. B: At the apex, even more and more disorganized rows of hair cells are seen. The polarity of hair cells is most profoundly disoriented in the outermost row of hair cells (top) where most cells are oriented in parallel to the long axis of the cochlea. GER, greater epithelial ridge; OHC, outer hair cells; IHC, inner hair cells. Scale bars = 100 μm in A; 10 μm in B–D.
Fig. 6
Innervation defects at embryonic day (E) 18.5. A: Injection of lipophilic tracers into the brain reliably labels fibers to all six sensory epithelia of the ear (insert). A,D: In the wild-type, there is innervation to the anterior and horizontal cristae and utricle (A) and the cochlea (D). B,C: In contrast, in Foxg1 null mutants, there are no labeled fibers to the remaining two canal cristae, the anterior (B,C) and posterior crista (insert in C). In contrast, the utricle, saccule, and cochlea are well innervated after carbocyanine dye injection into the brainstem (B,C). D: The wild-type cochlea shows three rows of outer and one row of inner hair cells densely innervated, with no fibers projecting beyond the organ of Corti. F: In contrast, innervation of the apical tip of the cochlea in Foxg1 null mutants shows the multiple rows of hair cells, outlined by numerous fibers extending beyond the multiple rows of hair cells, forming loops in the outer spiral sulcus. E: Innervation of the middle turn of the Foxg1 mutant is more clearly organized and shows prominent labeling of inner hair cells but poorly organized innervation to outer hair cells and is not organized into discrete rows of fibers (compare D and E). AC, anterior crista; HC, horizontal crista; PC, posterior crista; S, saccule; SpGl, spiral ganglion; IHCs, inner hair cells; OHCs, outer hair cells; OC, organ of Corti. Scale bars = 100 μm.
Fig. 7
Early development of innervation using tracers and acetylated tubulin staining. D: Wild-type mice have profound innervation to all vestibular sensory epithelia such as utricle, saccule, and posterior crista as early as embryonic day (E) 12.5, but show no innervation to the cochlea after cerebellar injections. A: In contrast, Foxg1 mutant littermates show only a sparse innervation to the utricle and saccule, no fibers at all to the canal cristae, but some fibers to the base of the cochlea. C,E: Of interest, labeling nerve fibers with tubulin shows, in Foxg1 mutant at E18.5, an innervation to the utricle and the anterior crista. B,E: The innervation overlaps with MYO VIIa positive hair cells (compare B and E). Note that these are fibers we were able to stain using tubulin but were unable to label using dyes injected into the brainstem. This finding suggests that theses fibers from the anterior crista do not project into the central nervous system and, thus, remain unlabeled with lipophilic tracers. Tubulin staining of the cochlea shows a more profound extension of fibers beyond the organ of Corti than lipophilic tracers do. F: Furthermore, there is a more profound innervation of the organ of Corti that also shows segregation into more densely innervated inner hair cell rows and less densely innervated outer hair cell rows. U, utricle; S, saccule; VGl, vestibular ganglion; CO, cochlea; AC, anterior crista; PC, posterior crista; OC, organ of Corti; SpGl, spiral ganglion. Scale bar = 100 μm.
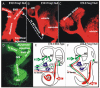
Fig. 8
A–F: Dye injections reveal connections between sensory epithelia in Foxg1 null mice. The differences between dye tracing after central injections and tubulin fiber labeling suggests that some sensory neurons projecting to the anterior crista do not project to the brain. To directly test this hypothesis, we injected different colored lipophilic dyes into the cochlea and anterior canal crista. E: In wild-type mice, such injections reliably label central projections and discrete populations of sensory neurons, but only some efferent fiber branches to other sensory epithelia. In contrast, dye injections into the cochlea of the Foxg1 mutant show not only fibers projecting to the brain but also labels sensory neurons in the vestibular ganglia as well as fibers entering the vestibular nerve to the anterior crista and utricle. A,B,C,F: These fibers can be traced to the utricle and anterior cristae. D,F: Injection of dye into the anterior crista with some labeling of the utricle (D) again labels vestibular neurons in both vestibular ganglia and fibers to the cochlea, including numerous fibers that extend beyond the organ of Corti (D,F). These data suggest that absence of Foxg1 alters the path-finding properties of canal sensory neurons, restricting their projection to the ear with no branches toward the brain. SVG, superior vestibular ganglion; IVG, inferior vestibular ganglion; AC, anterior crista; OC, organ of Corti; HC, horizontal canal; PC, posterior crista; U, utricle; S, saccule; C, cochlea. Scale bar = 100 μm.
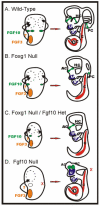
Fig. 9
Size of the sensory epithelia precursor determines size of the developing canal. Fgf10 and perhaps Fgf3 regulate growth of the canal plates in the wild-type ear. A: These signals are critical as early as embryonic day (E) 11.5. In the Foxg1 null, no horizontal crista forms. B: The horizontal canal formation likely relies on signals from the anterior crista. Reduction in the size of the posterior crista leads to smaller posterior canals in the Foxg1 null/Fgf10 heterozygote. In extreme cases, no posterior crista or canal forms. C: Note: in no case did we obtain a posterior canal without a posterior crista. The lack of Fgf10 expression in the Fgf10 null leads to complete lack of the posterior crista and canal. D: Partial formation of the anterior and horizontal cristae may be due to Fgf3 expression in the anterior part of the otocyst. This model allows us to predict the extent of canal formation based on size of the presumptive sensory epithelia. Compiled after Pauley et al. (2003), and Wright and Mansour (2003). AC, anterior crista; HC, horizontal crista; PC, posterior crista; U, utricle; S, saccule; C, cochlea.
Similar articles
- Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea.
Zhang S, Zhang Y, Dong Y, Guo L, Zhang Z, Shao B, Qi J, Zhou H, Zhu W, Yan X, Hong G, Zhang L, Zhang X, Tang M, Zhao C, Gao X, Chai R. Zhang S, et al. Cell Mol Life Sci. 2020 Apr;77(7):1401-1419. doi: 10.1007/s00018-019-03291-2. Epub 2019 Sep 4. Cell Mol Life Sci. 2020. PMID: 31485717 Free PMC article. - Foxg1 is required for proper separation and formation of sensory cristae during inner ear development.
Hwang CH, Simeone A, Lai E, Wu DK. Hwang CH, et al. Dev Dyn. 2009 Nov;238(11):2725-34. doi: 10.1002/dvdy.22111. Dev Dyn. 2009. PMID: 19842177 - Rbpj regulates development of prosensory cells in the mammalian inner ear.
Yamamoto N, Chang W, Kelley MW. Yamamoto N, et al. Dev Biol. 2011 May 15;353(2):367-79. doi: 10.1016/j.ydbio.2011.03.016. Epub 2011 Mar 21. Dev Biol. 2011. PMID: 21420948 - Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain.
Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Fritzsch B, et al. Hear Res. 2005 Aug;206(1-2):52-63. doi: 10.1016/j.heares.2004.11.025. Hear Res. 2005. PMID: 16080998 Free PMC article. Review. - Signaling regulating inner ear development: cell fate determination, patterning, morphogenesis, and defects.
Nakajima Y. Nakajima Y. Congenit Anom (Kyoto). 2015 Feb;55(1):17-25. doi: 10.1111/cga.12072. Congenit Anom (Kyoto). 2015. PMID: 25040109 Review.
Cited by
- Slc26a9P2ACre : a new CRE driver to regulate gene expression in the otic placode lineage and other FGFR2b-dependent epithelia.
Urness LD, Wang X, Li C, Quadros RM, Harms DW, Gurumurthy CB, Mansour SL. Urness LD, et al. Development. 2020 Jul 8;147(13):dev191015. doi: 10.1242/dev.191015. Development. 2020. PMID: 32541002 Free PMC article. - Intrinsic regenerative potential of murine cochlear supporting cells.
Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, Sinkkonen W, Cheng AG, Oshima K, Heller S. Sinkkonen ST, et al. Sci Rep. 2011;1:26. doi: 10.1038/srep00026. Epub 2011 Jun 29. Sci Rep. 2011. PMID: 22355545 Free PMC article. - Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea.
Zhang S, Zhang Y, Dong Y, Guo L, Zhang Z, Shao B, Qi J, Zhou H, Zhu W, Yan X, Hong G, Zhang L, Zhang X, Tang M, Zhao C, Gao X, Chai R. Zhang S, et al. Cell Mol Life Sci. 2020 Apr;77(7):1401-1419. doi: 10.1007/s00018-019-03291-2. Epub 2019 Sep 4. Cell Mol Life Sci. 2020. PMID: 31485717 Free PMC article. - The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear.
Hurd EA, Poucher HK, Cheng K, Raphael Y, Martin DM. Hurd EA, et al. Development. 2010 Sep;137(18):3139-50. doi: 10.1242/dev.047894. Development. 2010. PMID: 20736290 Free PMC article. - RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: implication of MEF2 and FOXM1 transcription factors.
Sanchez-Calderon H, Rodriguez-de la Rosa L, Milo M, Pichel JG, Holley M, Varela-Nieto I. Sanchez-Calderon H, et al. PLoS One. 2010 Jan 25;5(1):e8699. doi: 10.1371/journal.pone.0008699. PLoS One. 2010. PMID: 20111592 Free PMC article.
References
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner eardevelopment. Development. 2004;131:4119–4130. - PubMed
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. - PubMed
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs insensorycristae. Development. 2004;131:4201–4211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1 C06 RR17417-01/RR/NCRR NIH HHS/United States
- P20 RR018788/RR/NCRR NIH HHS/United States
- C06 RR017417/RR/NCRR NIH HHS/United States
- R01 DC005590/DC/NIDCD NIH HHS/United States
- 1P20RR018788-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases