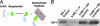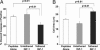Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction - PubMed (original) (raw)
Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction
Michael E Davis et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Strategies for cardiac repair include injection of cells, but these approaches have been hampered by poor cell engraftment, survival, and differentiation. To address these shortcomings for the purpose of improving cardiac function after injury, we designed self-assembling peptide nanofibers for prolonged delivery of insulin-like growth factor 1 (IGF-1), a cardiomyocyte growth and differentiation factor, to the myocardium, using a "biotin sandwich" approach. Biotinylated IGF-1 was complexed with tetravalent streptavidin and then bound to biotinylated self-assembling peptides. This biotin sandwich strategy allowed binding of IGF-1 but did not prevent self-assembly of the peptides into nanofibers within the myocardium. IGF-1 that was bound to peptide nanofibers activated Akt, decreased activation of caspase-3, and increased expression of cardiac troponin I in cardiomyocytes. After injection into rat myocardium, biotinylated nanofibers provided sustained IGF-1 delivery for 28 days, and targeted delivery of IGF-1 in vivo increased activation of Akt in the myocardium. When combined with transplanted cardiomyocytes, IGF-1 delivery by biotinylated nanofibers decreased caspase-3 cleavage by 28% and increased the myocyte cross-sectional area by 25% compared with cells embedded within nanofibers alone or with untethered IGF-1. Finally, cell therapy with IGF-1 delivery by biotinylated nanofibers improved systolic function after experimental myocardial infarction, demonstrating how engineering the local cellular microenvironment can improve cell therapy.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
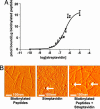
Fig. 1.
Streptavidin-binding to the biotinylated peptides. (A) Binding curve demonstrating the binding capacity of 35S-streptavidin to biotinylated self-assembling peptides. (B) AFM images of the biotinylated self-assembling peptide nanofibers alone (Left), streptavidin alone (Center), or streptavidin coupled to the biotinylated self-assembling peptide nanofibers (Right). Arrows show spherical streptavidin molecules.
Fig. 2.
Coupling to biotinylated self-assembling peptide nanofibers with the biotin sandwich approach. (A) Schematic showing the biotin sandwich method of tethering biotinylated IGF-1 (b-IGF-1) to biotinylated self-assembling peptides by using tetravalent streptavidin. This process does not interfere with self-assembly of peptides. (B) Representative Western blot analysis demonstrating tethering of biotinylated IGF-1 only to the biotinylated self-assembling peptides (b-IGF-1 + BP) and not the control nonbiotinylated peptides (b-IGF-1 + P). Premixing b-IGF-1 with biotinylated peptides vs. adding b-IGF-1 after self-assembly of biotinylated peptides occurred made no difference to b-IGF-1 binding.
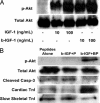
Fig. 3.
Bioactivity of biotin sandwich and effect on cardiomyocytes. (A) Representative Western blot analysis demonstrating equivalent abilities of IGF-1 and biotinylated IGF-1 sandwich (b-IGF-1) to cause phosphorylation of Akt. (B) Representative Western blot analyses demonstrating increased activation of the survival factor Akt, decreased activation of caspase-3, and increased maturation markers [increased cardiac troponin I (TnI) and decreased slow skeletal troponin I] with tethered b-IGF-1 coupled with streptavidin in biotinylated peptides (b-IGF-1 + BP) compared with peptides alone and untethered b-IGF-1 coupled with streptavidin with control nonbiotinylated peptides (b-IGF-1 + P).
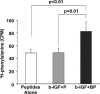
Fig. 4.
Effect of tethered IGF-1 on new protein synthesis. Increase in cardiomyocyte [3H]phenylalanine incorporation after 14 days with tethered IGF-1 coupled with streptavidin in biotinylated peptide nanofibers (b-IGF-1 + BP) compared with peptide nanofibers alone and untethered IGF-1 coupled with streptavidin with control nonbiotinylated peptide nanofibers (b-IGF-1 + P).
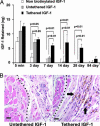
Fig. 5.
In vivo delivery and bioactivity of tethered IGF-1. (A) Quantitative delivery of human IGF-1 to the myocardium of rats after 5 min and 7, 14, 28, and 84 days. Twenty-five nanograms of free nonbiotinylated IGF-1 (negative control), biotinylated IGF-1 bound to streptavidin in nonbiotinylated peptides (untethered b-IGF-1; negative control), or tethered b-IGF-1 with streptavidin coupled to biotinylated self-assembling peptides was injected into the myocardium of rats (n ≥ 4 for each group per time point). (B) Immunohistochemistry showing phospho-Akt-specific staining in untethered b-IGF-1 (Left) and tethered b-IGF-1 (Right) samples. Arrows denote areas staining positive for phospho-Akt (brown staining). (Scale bars: 20 μm.)
Fig. 6.
Effects of tethered IGF-1 on survival and growth of implanted cells. Effects of tethered b-IGF-1 on implanted neonatal cardiac myocytes. Decreased cleavage of caspase-3 (A) and increased size (B) were found in cells embedded within tethered b-IGF-1 self-assembling peptides after myocardial implantation.
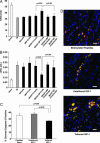
Fig. 7.
Tethered IGF-1 improves efficacy of cell implantation on cardiac function after myocardial infarction. (A) Improvement in fractional shortening after 21 days in cells embedded within tethered b-IGF-1 containing self-assembling peptides (MI/IGF+BP) compared with untethered b-IGF containing self-assembling peptides (MI/IGF+P) and dominant negative transduced cells with tethered b-IGF-1 (MI/IGF+BP+Cells/dnAkt). (B) Ventricular dilation as measured by the increase in ventricular volume from 1 day to 21 days after infarction was not observed in rats treated with cells embedded within self-assembling peptides containing tethered b-IGF-1. (C) Cleavage of caspase-3 in implanted cells was significantly reduced in the presence of tethered IGF-1, whereas untethered IGF-1 had no effect. (D) Representative immunofluorescent staining showing cell size in treatment groups. GFP-positive cells (green) costained with tropomyosin (red) in peptides alone (Top), untethered (Middle), and tethered (Bottom) b-IGF-1 sections. Blue stain is DAPI (nuclei). (Scale bars: 20 μm.)
Similar articles
- Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction.
Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Padin-Iruegas ME, et al. Circulation. 2009 Sep 8;120(10):876-87. doi: 10.1161/CIRCULATIONAHA.109.852285. Epub 2009 Aug 24. Circulation. 2009. PMID: 19704095 Free PMC article. - Paracrine Engineering of Human Cardiac Stem Cells With Insulin-Like Growth Factor 1 Enhances Myocardial Repair.
Jackson R, Tilokee EL, Latham N, Mount S, Rafatian G, Strydhorst J, Ye B, Boodhwani M, Chan V, Ruel M, Ruddy TD, Suuronen EJ, Stewart DJ, Davis DR. Jackson R, et al. J Am Heart Assoc. 2015 Sep 11;4(9):e002104. doi: 10.1161/JAHA.115.002104. J Am Heart Assoc. 2015. PMID: 26363004 Free PMC article. - Transplantation of marrow-derived cardiac stem cells carried in designer self-assembling peptide nanofibers improves cardiac function after myocardial infarction.
Guo HD, Cui GH, Wang HJ, Tan YZ. Guo HD, et al. Biochem Biophys Res Commun. 2010 Aug 13;399(1):42-8. doi: 10.1016/j.bbrc.2010.07.031. Epub 2010 Jul 15. Biochem Biophys Res Commun. 2010. PMID: 20637726 - Self-assembling peptide-based delivery of therapeutics for myocardial infarction.
French KM, Somasuntharam I, Davis ME. French KM, et al. Adv Drug Deliv Rev. 2016 Jan 15;96:40-53. doi: 10.1016/j.addr.2015.04.023. Epub 2015 May 7. Adv Drug Deliv Rev. 2016. PMID: 25959427 Review. - Nanofibers based tissue engineering and drug delivery approaches for myocardial regeneration.
Joshi J, Kothapalli CR. Joshi J, et al. Curr Pharm Des. 2015;21(15):2006-20. doi: 10.2174/1381612821666150302153138. Curr Pharm Des. 2015. PMID: 25732660 Review.
Cited by
- Stem-cell angiogenesis and regeneration of the heart: review of a saga of 2 decades.
Liebson PR. Liebson PR. Clin Cardiol. 2015 May;38(5):309-16. doi: 10.1002/clc.22381. Epub 2015 May 8. Clin Cardiol. 2015. PMID: 25955103 Free PMC article. Review. - In situ cardiac regeneration by using neuropeptide substance P and IGF-1C peptide eluting heart patches.
Shafiq M, Zhang Y, Zhu D, Zhao Z, Kim DH, Kim SH, Kong D. Shafiq M, et al. Regen Biomater. 2018 Oct;5(5):303-316. doi: 10.1093/rb/rby021. Epub 2018 Oct 12. Regen Biomater. 2018. PMID: 30338128 Free PMC article. - Theranostic Nanomedicines for the Treatment of Cardiovascular and Related Diseases: Current Strategies and Future Perspectives.
Manners N, Priya V, Mehata AK, Rawat M, Mohan S, Makeen HA, Albratty M, Albarrati A, Meraya AM, Muthu MS. Manners N, et al. Pharmaceuticals (Basel). 2022 Apr 1;15(4):441. doi: 10.3390/ph15040441. Pharmaceuticals (Basel). 2022. PMID: 35455438 Free PMC article. Review. - Nanoscale Self-Assembly for Therapeutic Delivery.
Yadav S, Sharma AK, Kumar P. Yadav S, et al. Front Bioeng Biotechnol. 2020 Feb 25;8:127. doi: 10.3389/fbioe.2020.00127. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32158749 Free PMC article. Review. - Degradable acetalated dextran microparticles for tunable release of an engineered hepatocyte growth factor fragment.
Suarez SL, Muñoz A, Mitchell A, Braden RL, Luo C, Cochran JR, Almutairi A, Christman KL. Suarez SL, et al. ACS Biomater Sci Eng. 2016 Feb 8;2(2):197-204. doi: 10.1021/acsbiomaterials.5b00335. Epub 2015 Dec 14. ACS Biomater Sci Eng. 2016. PMID: 29333489 Free PMC article.
References
- Menasche P. Ann. Thorac. Surg. 2003;75:S20–S28. - PubMed
- Menasche P. Curr. Opin. Cardiol. 2004;19:154–161. - PubMed
- Mangi A. A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J. S., Dzau V. J. Nat. Med. 2003;9:1195–1201. - PubMed
- Palmen M., Daemen M. J., Bronsaer R., Dassen W. R., Zandbergen H. R., Kockx M., Smits J. F., van der Zee R., Doevendans P. A. Cardiovasc. Res. 2001;50:516–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous