Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies - PubMed (original) (raw)
Review
Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies
Edwin Li et al. Biochemistry. 2006.
Abstract
Receptor tyrosine kinases (RTKs) conduct biochemical signals via lateral dimerization in the plasma membrane, and their transmembrane (TM) domains play an important role in the dimerization process. Here we present two models of RTK-mediated signaling, and we discuss the role of the TM domains within the framework of these two models. We summarize findings of single-amino acid mutations in RTK TM domains that induce unregulated signaling and, as a consequence, pathological phenotypes. We review the current knowledge of pathology induction mechanisms due to these mutations, focusing on the structural and thermodynamic basis of pathogenic dimer stabilization.
Figures
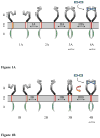
Figure 1
Two models of RTK-mediated signaling. The difference between the two models is in the conformation and activity of the unliganded dimer. In model A, the unliganded dimer (3A) is active. The ligand (blue) stabilizes the dimer, but does not change the dimer structure (4A). In model B, the unliganded dimer (3B) is inactive. Ligand binding induces a structural change to an active dimer (4B). EC: extracellular domain. TM: transmembrane domain. IC: intracellular catalytic domain.
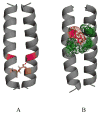
Figure 2
Pathogenic interactions, believed to contribute to the stability of mutant RTK TM domain dimers. (A) A putative hydrogen bond between Glu391 (orange) and Ile387 (magenta) in the pathogenic Ala391Glu FGFR3 mutant, linked to Crouzon syndrome with acanthosis nigricans and bladder cancer (86). (B) Putative cation-π interactions between Arg380 (red) and aromatic residues (green) in the achondroplasia-causing Gly380Arg FGFR3 mutant. These cation-π interactions likely compensate for the electrostatic repulsion between the two arginines in the mutant dimer. The comparison of these two mutant structures with the wild-type FGFR3 TM dimer structure (86) shows that the mutations induce modest changes in the relative orientation of the two helices close to the C-terminus (not discussed here in detail). Thus, the orientation of the catalytic domains is likely not perturbed due to the mutations, inaccordance with the structural hypothesis discussed here.
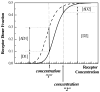
Figure 3
The increase in receptor dimer fraction due to a pathogenic mutation depends of three parameters: (1) the difference in dimerization propensities between wild-type and mutant receptors, (2) the dimerization propensity of the wild-type receptor, which in turn depends on the ligand concentration, and (3) the receptor concentration in the plasma membrane. The functional dependence of the receptor dimer fraction on receptor concentration in the membrane is shown for an arbirary dimerization propensity of the wild-type receptor (solid line). The dashed line depicts a higher dimer fraction due to a pathogenic mutation (86). The figure illustrates that the relative increase in dimer fraction [ΔD]/[D], where [D] is the wild-type dimer fraction and [ΔD] is the increase due to the mutation, depends on the receptor concentration in the membrane. For example, the relative increase in dimer fraction [ΔD1]/[D1] for concentration “1” is [ΔD1]/[D1] ~ 2, such that the dimer fraction increases 3- fold due to the mutation. However, a 100-fold increase in expression level will result in only about 10% increase in dimer fraction ([ΔD2]/[D2] ~ 0.1 for concentration “2”) due to the same mutation. Note that the dimerization propensity of the wild-type receptors in the plasma membrane, determining the exact position of the solid line, is yet to be characterized (the difference between the solid and the dashed line correspond to −1.3 kcal/mole (86)). This is a challenging task since the dynamic monomer-dimer distribution in the plasma membrane is expected to depend on the concentration of the available ligand (86), but may be accomplished in the future using FRET. Until this is accomplished, the dependence of the receptor dimer fraction on the expression level, as well as the increase in receptor dimer fraction due to pathogenic mutations, cannot be predicted quantitatively.
Similar articles
- Receptor tyrosine kinase transmembrane domains: Function, dimer structure and dimerization energetics.
Li E, Hristova K. Li E, et al. Cell Adh Migr. 2010 Apr-Jun;4(2):249-54. doi: 10.4161/cam.4.2.10725. Epub 2010 Apr 23. Cell Adh Migr. 2010. PMID: 20168077 Free PMC article. Review. - Synthesis and initial characterization of FGFR3 transmembrane domain: consequences of sequence modifications.
Iwamoto T, You M, Li E, Spangler J, Tomich JM, Hristova K. Iwamoto T, et al. Biochim Biophys Acta. 2005 Mar 1;1668(2):240-7. doi: 10.1016/j.bbamem.2004.12.012. Epub 2005 Jan 28. Biochim Biophys Acta. 2005. PMID: 15737335 - FGFR3 dimer stabilization due to a single amino acid pathogenic mutation.
Li E, You M, Hristova K. Li E, et al. J Mol Biol. 2006 Feb 24;356(3):600-12. doi: 10.1016/j.jmb.2005.11.077. Epub 2005 Dec 12. J Mol Biol. 2006. PMID: 16384584 Free PMC article. - Studies of receptor tyrosine kinase transmembrane domain interactions: the EmEx-FRET method.
Merzlyakov M, Chen L, Hristova K. Merzlyakov M, et al. J Membr Biol. 2007 Feb;215(2-3):93-103. doi: 10.1007/s00232-007-9009-0. Epub 2007 Jun 14. J Membr Biol. 2007. PMID: 17565424 Free PMC article. - Autoinhibitory mechanisms in receptor tyrosine kinases.
Hubbard SR. Hubbard SR. Front Biosci. 2002 Feb 1;7:d330-40. doi: 10.2741/A778. Front Biosci. 2002. PMID: 11815286 Review.
Cited by
- Structural analysis of genomic and proteomic signatures reveal dynamic expression of intrinsically disordered regions in breast cancer.
Zatorski N, Sun Y, Elmas A, Dallago C, Karl T, Stein D, Rost B, Huang KL, Walsh M, Schlessinger A. Zatorski N, et al. iScience. 2024 Aug 7;27(9):110640. doi: 10.1016/j.isci.2024.110640. eCollection 2024 Sep 20. iScience. 2024. PMID: 39310778 Free PMC article. - Effect of the G375C and G346E achondroplasia mutations on FGFR3 activation.
He L, Serrano C, Niphadkar N, Shobnam N, Hristova K. He L, et al. PLoS One. 2012;7(4):e34808. doi: 10.1371/journal.pone.0034808. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529939 Free PMC article. - Oligomerization of the transmembrane domain of IRE1α in SDS micelles.
Cho H, Lamarca R, Chan C. Cho H, et al. Biochem Biophys Res Commun. 2012 Nov 2;427(4):764-7. doi: 10.1016/j.bbrc.2012.09.135. Epub 2012 Oct 4. Biochem Biophys Res Commun. 2012. PMID: 23041190 Free PMC article. - Structural basis of the transmembrane domain dimerization and rotation in the activation mechanism of the TRKA receptor by nerve growth factor.
Franco ML, Nadezhdin KD, Goncharuk SA, Mineev KS, Arseniev AS, Vilar M. Franco ML, et al. J Biol Chem. 2020 Jan 3;295(1):275-286. doi: 10.1074/jbc.RA119.011312. Epub 2019 Dec 4. J Biol Chem. 2020. PMID: 31801826 Free PMC article. - A classification of genes involved in normal and delayed male puberty.
Akram M, Raza Rizvi SS, Qayyum M, Handelsman DJ. Akram M, et al. Asian J Androl. 2023 Mar-Apr;25(2):230-239. doi: 10.4103/aja202210. Asian J Androl. 2023. PMID: 35532554 Free PMC article. Review.
References
- Fantl WJ, Johnson DE, Williams LT. Signaling by Receptor Tyrosine Kinases. Annu Rev Biochem. 1993;62:453–481. - PubMed
- van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. - PubMed
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. - PubMed
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. - PubMed
- Robertson SC, Tynan JA, Donoghue DJ. RTK mutations and human syndromes - when good receptors turn bad. Trends Genet. 2000;16:265–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources