NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes - PubMed (original) (raw)
NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes
Fabrizia Stavru et al. J Cell Biol. 2006.
Abstract
POM121 and gp210 were, until this point, the only known membrane-integral nucleoporins (Nups) of vertebrates and, thus, the only candidate anchors for nuclear pore complexes (NPCs) within the nuclear membrane. In an accompanying study (Stavru et al.), we provided evidence that NPCs can exist independently of POM121 and gp210, and we predicted that vertebrate NPCs contain additional membrane-integral constituents. We identify such an additional membrane protein in the NPCs of mammals, frogs, insects, and nematodes as the orthologue to yeast Ndc1p/Cut11p. Human NDC1 (hNDC1) likely possesses six transmembrane segments, and it is located at the nuclear pore wall. Depletion of hNDC1 from human HeLa cells interferes with the assembly of phenylalanine-glycine repeat Nups into NPCs. The loss of NDC1 function in Caenorhabditis elegans also causes severe NPC defects and very high larval and embryonic mortality. However, it is not ultimately lethal. Instead, homozygous NDC1-deficient worms can be propagated. This indicates that none of the membrane-integral Nups is universally essential for NPC assembly, and suggests that NPC biogenesis is an extremely fault-tolerant process.
Figures
Figure 1.
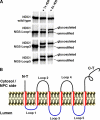
Multiple alignment of selected NDC1 orthologues. Predicted TMSs (1–6) are indicated in red, luminal loops are indicated in blue, and cytoplasmic parts remained uncolored. Cytoplasmic localization of the NH2 and COOH termini, as well as the luminal localization of loops 1, 3, and 5, is supported by experimental evidence (see Fig. 3 and Fig. 4 A, as well as Fig. S3). Residues were shaded in black when identical and in gray when similar in >40% of the sequences. hs, H. sapiens; xl, X. laevis; dm1 and dm2, D. melanogaster NDC1 variants 1 and 2; ce, C. elegans; sc, S. cerevisiae; sp, S. pombe; nc, N. crassa; at, A. thaliana; cn, C. neoformans. Fig. S3 is available at
http://www.jcb.org/cgi/content/full/jcb.200601001/DC1
.
Figure 2.
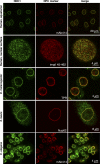
Animal NDC1 proteins localize to NPCs. Antibodies were raised against NDC1 from H. sapiens, X. laevis, D. melanogaster (variant 1), and C. elegans, and then affinity purified and used to localize NDC1 in human HeLa cells, D. melanogaster Schneider cells, X. laevis XL177 cells, or cells isolated from C. elegans. The following NPC markers were used: mAb414, antibodies against D. melanogaster TPR, anti–X. laevis Nup62, and the fluorescently labeled importin β45–462 fragment. Optical sections through the nuclear equator and the nuclear surface are shown.
Figure 3.
Membrane topology of endogenous hNDC1 probed with anti-hNDC1 antibodies. (A) HeLa cells were fixed with paraformaldehyde and permeabilized with 0.05% digitonin, which perforates the plasma membrane but leaves ER and NE intact. Nuclear or luminal epitopes, hence, remain inaccessible for antibodies. Yet, antibodies clearly recognized the COOH-terminal domain of NDC1 under these conditions (top). The COOH terminus of NDC1 is, therefore, extraluminal, i.e., exposed to cytoplasm or the central pore channel. The procedure was verified by two internal controls; as expected, mAb414 also recognized NPCs from the cytoplasmic side, whereas recognition of TPR, which is located on the nuclear NPC side, required complete permeabilization of internal membranes by 0.25% Triton X-100. Detection of bound primary antibodies was carried out with Alexa Fluor 488 anti–rabbit (for anti-NDC1), Alexa Fluor 647 anti–mouse (for mAb414), and Alexa Fluor 568 anti–guinea pig (for anti-TPR). Images were taken by confocal laser scanning microscopy. (B) Analysis was analogous to Fig. 3 A, the difference being that an antibody against loop 5 of NDC1 was used. Loop 5 is inaccessible from the cytoplasm and recognized by the antibody only after dissolving internal lipid bilayers with Triton X-100.
Figure 4.
hNDC topology probed by N-glycosylation site tagging. (A) Indicated forms of hNDC1 were translated in vitro in the presence of [35S]methionine and signal recognition particles, and subsequently detected by autoradiography. NGSs introduced into loops 1, 3, or 5 were indeed efficiently glycosylated, provided that RER membranes were present during translation. As N-glycosylation activity is RER-luminal, loops 1, 3, and 5 must also be luminal. (B) Scheme of the proposed membrane topology of human NDC1.
Figure 5.
hNDC1 is a central component of NPCs. (A) An immunoblot of total HeLa cells probed with affinity-purified antibodies raised against the COOH-terminal domain of hNDC1 (hNDC1292–674). Typical of a multimembrane-spanning protein, hNDC1 migrates faster on SDS-gels than expected from its calculated molecular mass (76 kD). (B) HeLa cells were fixed in 2% formaldehyde + 0.1% glutaraldehyde, embedded into LR white and processed for postembedding immunogold-labeling as described previously (Krull et al., 2004). hNDC1 was detected by the antibodies characterized in A. Arrows in the representative EM images point at two neighboring NPCs in cross section. Bar, 50 nm. C, cytoplasmic; N, nuclear side of the NE.
Figure 6.
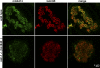
Depletion of hNDC1 from HeLa cells causes severe NPC assembly defects. HeLa cells were transfected with hNDC1-specific siRNAs and analyzed 96 h later by double immunofluorescence with indicated antibody combinations. Depletion of hNDC1 from transfected cells (arrows) resulted in a proportional decrease in mAb414 and Nup88 stain.
Figure 7.
Immunoblot analysis of the ndc-1 −_/_− (tm1845) C. elegans mutant. Total protein extracts from wild-type worms, the homozygous ndc-1(tm1845) strain, and ceNDC1∷GFP rescue strain were prepared and analyzed by immunoblotting with affinity-purified antibodies against ceNDC-1 and antibodies against lamin B, which served as a loading control.
Figure 8.
Reduced mAb414 signal at NPCs of homozygous ndc-1 −_/_− (tm1845) mutant C. elegans embryos. Wild-type embryos or embryos lacking ceNDC-1 were stained in parallel with mAb414 (green) and anti-laminB (red). Note that NPCs of the mutant embryos stain only weakly with the NPC marker mAb414. The increased mAb414 signal outside the NE suggests that a significant proportion of FG repeat Nups failed to assemble into NPCs. The smaller size and altered morphology of the mutant embryos is probably a secondary effect of impaired NPC function.
Figure 9.
Localization of the ceNDC1∷GFP rescue protein. Figure shows analysis of the homozygous ndc-1 −_/_− (tm1845) strain rescued by an extrachromosomal array that allows expression of a ceNDC-1∷GFP fusion. Adult hermaphrodites were mounted on a slide and imaged by differential interference contrast or conventional fluorescence microscopy in the GFP channel. The NDC-1∷GFP fusion shows the expected NPC stain. However, because of the extrachromosomal array, it is not expressed in every cell.
Comment in
- Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells.
Stavru F, Nautrup-Pedersen G, Cordes VC, Görlich D. Stavru F, et al. J Cell Biol. 2006 May 22;173(4):477-83. doi: 10.1083/jcb.200601002. Epub 2006 May 15. J Cell Biol. 2006. PMID: 16702234 Free PMC article.
Similar articles
- The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells.
Mansfeld J, Güttinger S, Hawryluk-Gara LA, Panté N, Mall M, Galy V, Haselmann U, Mühlhäusser P, Wozniak RW, Mattaj IW, Kutay U, Antonin W. Mansfeld J, et al. Mol Cell. 2006 Apr 7;22(1):93-103. doi: 10.1016/j.molcel.2006.02.015. Mol Cell. 2006. PMID: 16600873 - Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells.
Stavru F, Nautrup-Pedersen G, Cordes VC, Görlich D. Stavru F, et al. J Cell Biol. 2006 May 22;173(4):477-83. doi: 10.1083/jcb.200601002. Epub 2006 May 15. J Cell Biol. 2006. PMID: 16702234 Free PMC article. - The nuclear pore complex protein ALADIN is anchored via NDC1 but not via POM121 and GP210 in the nuclear envelope.
Kind B, Koehler K, Lorenz M, Huebner A. Kind B, et al. Biochem Biophys Res Commun. 2009 Dec 11;390(2):205-10. doi: 10.1016/j.bbrc.2009.09.080. Epub 2009 Sep 24. Biochem Biophys Res Commun. 2009. PMID: 19782045 - [Nuclear pores: from yeast to higher eukaryotes].
Doye V. Doye V. J Soc Biol. 2002;196(4):349-54. J Soc Biol. 2002. PMID: 12645306 Review. French. - Function and assembly of nuclear pore complex proteins.
Bodoor K, Shaikh S, Enarson P, Chowdhury S, Salina D, Raharjo WH, Burke B. Bodoor K, et al. Biochem Cell Biol. 1999;77(4):321-9. Biochem Cell Biol. 1999. PMID: 10546895 Review.
Cited by
- Comparative genomic evidence for a complete nuclear pore complex in the last eukaryotic common ancestor.
Neumann N, Lundin D, Poole AM. Neumann N, et al. PLoS One. 2010 Oct 8;5(10):e13241. doi: 10.1371/journal.pone.0013241. PLoS One. 2010. PMID: 20949036 Free PMC article. - The three fungal transmembrane nuclear pore complex proteins of Aspergillus nidulans are dispensable in the presence of an intact An-Nup84-120 complex.
Liu HL, De Souza CP, Osmani AH, Osmani SA. Liu HL, et al. Mol Biol Cell. 2009 Jan;20(2):616-30. doi: 10.1091/mbc.e08-06-0628. Epub 2008 Nov 19. Mol Biol Cell. 2009. PMID: 19019988 Free PMC article. - Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance.
Onischenko E, Stanton LH, Madrid AS, Kieselbach T, Weis K. Onischenko E, et al. J Cell Biol. 2009 May 4;185(3):475-91. doi: 10.1083/jcb.200810030. J Cell Biol. 2009. PMID: 19414609 Free PMC article. - NDC1 is a Prognostic Biomarker and Associated with Immune Infiltrates in Colon Cancer.
Liu M, Yuan R, Liu S, Xue Y, Wang X. Liu M, et al. Int J Gen Med. 2021 Nov 25;14:8811-8817. doi: 10.2147/IJGM.S325720. eCollection 2021. Int J Gen Med. 2021. PMID: 34858049 Free PMC article. - Compositionally distinct nuclear pore complexes of functionally distinct dimorphic nuclei in the ciliate Tetrahymena.
Iwamoto M, Osakada H, Mori C, Fukuda Y, Nagao K, Obuse C, Hiraoka Y, Haraguchi T. Iwamoto M, et al. J Cell Sci. 2017 May 15;130(10):1822-1834. doi: 10.1242/jcs.199398. Epub 2017 Apr 6. J Cell Sci. 2017. PMID: 28386019 Free PMC article.
References
- Aitchison, J.D., M.P. Rout, M. Marelli, G. Blobel, and R.W. Wozniak. 1995. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 131:1133–1148. - PMC - PubMed
- Antonin, W., C. Franz, U. Haselmann, C. Antony, and I.W. Mattaj. 2005. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol. Cell. 17:83–92. - PubMed
- Araki, Y., C.K. Lau, H. Maekawa, S.L. Jaspersen, T.H. Giddings Jr., E. Schiebel, and M. Winey. 2006. The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol. Biol. Cell. 17:1959–1970. - PMC - PubMed
- Bodoor, K., S. Shaikh, D. Salina, W.H. Raharjo, R. Bastos, M. Lohka, and B. Burke. 1999. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J. Cell Sci. 112:2253–2264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials