Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells - PubMed (original) (raw)
Comment
. 2006 May 22;173(4):477-83.
doi: 10.1083/jcb.200601002. Epub 2006 May 15.
Affiliations
- PMID: 16702234
- PMCID: PMC2063858
- DOI: 10.1083/jcb.200601002
Comment
Nuclear pore complex assembly and maintenance in POM121- and gp210-deficient cells
Fabrizia Stavru et al. J Cell Biol. 2006.
Abstract
So far, POM121 and gp210 are the only known anchoring sites of vertebrate nuclear pore complexes (NPCs) within the lipid bilayer of the nuclear envelope (NE) and, thus, are excellent candidates for initiating the NPC assembly process. Indeed, we demonstrate that POM121 can recruit several nucleoporins, such as Nup62 or Nup358, to ectopic assembly sites. It thus appears to act as a nucleation site for the assembly of NPC substructures. Nonetheless, we observed functional NPCs and intact NEs in severely POM121-depleted cells. Double knockdowns of gp210 and POM121 in HeLa cells, as well as depletion of POM121 from human fibroblasts, which do not express gp210, further suggest that NPCs can assemble or at least persist in a POM121- and gp210-free form. This points to extensive redundancies in protein-protein interactions within NPCs and suggests that vertebrate NPCs contain additional membrane-integral nucleoporins for anchorage within the lipid bilayer of the NE. In Stavru et al., we describe such an additional transmembrane nucleoporin as the metazoan orthologue of yeast Ndc1p.
Figures
Figure 1.
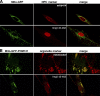
Ectopic expression of POM121 at mitochondria. (A) EGFP was fused behind residues 1–70 of TOM20, expressed in HeLa cells, and thereby anchored to the outer mitochondrial membrane (Mito-GFP). (top) Colocalization of the fusion protein with the NPC marker mAb414. (bottom) Staining of NPCs with the Alexa 568–labeled Impβ45–462 after digitonin permeabilization. Signals for mitochondria and NPCs do not overlap. (B) The POM12173–797 fragment, lacking its natural membrane anchor and the FG repeat domain, was fused behind the Mito-GFP module (Mito-GFP-POM121) and expressed in HeLa cells. (top) Colocalization of the Mito-GFP-POM121 signal with mitotracker-stained mitochondria (images depict a transfected and a nontransfected cell). (bottom) Bright staining with Impβ45–462 indicates recruitment of FG or GLFG repeat Nups to the ectopic POM121 fragment at mitochondria. Clustering of mitochondria is a side effect of this ectopic expression. The weak Mito-GFP-POM121 signal at the NE reflects the fact that targeting of the fusion protein to mitochondria is in competition with incorporation into bona fide NPCs.
Figure 2.
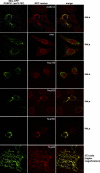
The central POM121 domain is sufficient to recruit a subset of Nups. Images show immunofluorescence colocalization of Mito-GFP-POM12173–797 with indicated NPC markers. The ectopic assembly sites clearly recruited FG-repeat Nups (detected by mAb414) and stained positive for Nup358, Nup205, and Nup62 but remained negative for Nup153 and TPR. As indicated, expression was either in HeLa cells or in 3T3 fibroblasts, whose mitochondria show less clustering upon Mito-GFP-POM12173–797 expression.
Figure 3.
POM121 is not limiting for NPC assembly. HeLa cells were transfected with POM121-specific siRNAs and fixed 60 h later. Triple immunofluorescences were performed with combinations of rabbit anti-POM121; mouse mAb414 or anti-Nup88; and guinea pig anti-Nup107, -Nup96, -Nup62, or -Nup358. Bright NE staining for POM121 is visible only in nontransfected cells that are shown for reference. POM121-depleted NPCs still contained wild-type levels of Nup107, Nup88, Nup358, Nup96, and Nup62 and stained normally with mAb414. White arrows indicate cells with <5% residual POM121. Red arrows label a virtually POM121-free pair of cells that just completed cytokinesis.
Figure 4.
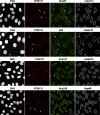
Assembly of human NPCs in the absence of gp210. (A) Primary neonatal fibroblasts (5 × 104 cells) and HeLa cells (2.5 × 104 cells) were analyzed by immunoblotting with the indicated antibodies. Note that fibroblasts appear gp210 negative. (B) HeLa cells were transfected with a gp210-specific siRNA and fixed 60 h later. DNA was stained with Hoechst 33342. Triple immunofluorescence was with rabbit anti-gp210, guinea pig anti-Nup107, and mouse mAb414. Arrows exemplify a transfected cell with <5% residual gp210. The gp210 depletion did not impair assembly of Nup107 or mAb414-reactive Nups into NPCs.
Figure 5.
NPCs appear normal even in cells lacking gp210 and depleted of POM121. (A) HeLa cells were treated simultaneously with POM121- and gp210-specific siRNAs and fixed 60 h later. Triple immunofluorescence was with Alexa 647–labeled anti-POM121, Alexa 488 anti-Nup107, and Alexa 568 anti-gp210. Arrows point to cells almost completely depleted of gp210 and POM121. Comparison with nontransfected cells indicates that this codepletion affected neither NE assembly nor assembly of Nup107 into NPCs. (B) POM121 was depleted by RNAi from human primary fibroblasts that already lack gp210 (Fig. 4 A). Arrows indicate cells whose POM121 staining was reduced to background levels. NE or NPC assembly remained unaffected as judged by their normal mAb414 signal.
Comment on
- NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes.
Stavru F, Hülsmann BB, Spang A, Hartmann E, Cordes VC, Görlich D. Stavru F, et al. J Cell Biol. 2006 May 22;173(4):509-19. doi: 10.1083/jcb.200601001. Epub 2006 May 15. J Cell Biol. 2006. PMID: 16702233 Free PMC article.
Similar articles
- NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes.
Stavru F, Hülsmann BB, Spang A, Hartmann E, Cordes VC, Görlich D. Stavru F, et al. J Cell Biol. 2006 May 22;173(4):509-19. doi: 10.1083/jcb.200601001. Epub 2006 May 15. J Cell Biol. 2006. PMID: 16702233 Free PMC article. - Dynamic properties of nuclear pore complex proteins in gp210 deficient cells.
Eriksson C, Rustum C, Hallberg E. Eriksson C, et al. FEBS Lett. 2004 Aug 13;572(1-3):261-5. doi: 10.1016/j.febslet.2004.07.044. FEBS Lett. 2004. PMID: 15304359 - The nuclear pore complex protein ALADIN is anchored via NDC1 but not via POM121 and GP210 in the nuclear envelope.
Kind B, Koehler K, Lorenz M, Huebner A. Kind B, et al. Biochem Biophys Res Commun. 2009 Dec 11;390(2):205-10. doi: 10.1016/j.bbrc.2009.09.080. Epub 2009 Sep 24. Biochem Biophys Res Commun. 2009. PMID: 19782045 - Function and assembly of nuclear pore complex proteins.
Bodoor K, Shaikh S, Enarson P, Chowdhury S, Salina D, Raharjo WH, Burke B. Bodoor K, et al. Biochem Cell Biol. 1999;77(4):321-9. Biochem Cell Biol. 1999. PMID: 10546895 Review. - Nucleoporins: leaving the nuclear pore complex for a successful mitosis.
Chatel G, Fahrenkrog B. Chatel G, et al. Cell Signal. 2011 Oct;23(10):1555-62. doi: 10.1016/j.cellsig.2011.05.023. Epub 2011 Jun 13. Cell Signal. 2011. PMID: 21683138 Review.
Cited by
- Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer.
Wurlitzer M, Möckelmann N, Kriegs M, Vens M, Omidi M, Hoffer K, Bargen CV, Möller-Koop C, Witt M, Droste C, Oetting A, Petersen H, Busch CJ, Münscher A, Schlüter H, Clauditz TS, Rieckmann T. Wurlitzer M, et al. Cancers (Basel). 2020 Jun 11;12(6):1531. doi: 10.3390/cancers12061531. Cancers (Basel). 2020. PMID: 32545200 Free PMC article. - Atypical mitochondrial fission upon bacterial infection.
Stavru F, Palmer AE, Wang C, Youle RJ, Cossart P. Stavru F, et al. Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16003-8. doi: 10.1073/pnas.1315784110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043775 Free PMC article. - Nuclear transport proteins: structure, function, and disease relevance.
Yang Y, Guo L, Chen L, Gong B, Jia D, Sun Q. Yang Y, et al. Signal Transduct Target Ther. 2023 Nov 10;8(1):425. doi: 10.1038/s41392-023-01649-4. Signal Transduct Target Ther. 2023. PMID: 37945593 Free PMC article. Review. - NDC1: a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes.
Stavru F, Hülsmann BB, Spang A, Hartmann E, Cordes VC, Görlich D. Stavru F, et al. J Cell Biol. 2006 May 22;173(4):509-19. doi: 10.1083/jcb.200601001. Epub 2006 May 15. J Cell Biol. 2006. PMID: 16702233 Free PMC article. - Nuclear pore complexes - a doorway to neural injury in neurodegeneration.
Coyne AN, Rothstein JD. Coyne AN, et al. Nat Rev Neurol. 2022 Jun;18(6):348-362. doi: 10.1038/s41582-022-00653-6. Epub 2022 Apr 29. Nat Rev Neurol. 2022. PMID: 35488039 Free PMC article. Review.
References
- Antonin, W., C. Franz, U. Haselmann, C. Antony, and I.W. Mattaj. 2005. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol. Cell. 17:83–92. - PubMed
- Berrios, M., V.H. Meller, M. McConnell, and P.A. Fisher. 1995. Drosophila gp210, an invertebrate nuclear pore complex glycoprotein. Eur. J. Cell Biol. 67:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous