The chemokine SDF-1/CXCL12 modulates the firing pattern of vasopressin neurons and counteracts induced vasopressin release through CXCR4 - PubMed (original) (raw)
The chemokine SDF-1/CXCL12 modulates the firing pattern of vasopressin neurons and counteracts induced vasopressin release through CXCR4
Céline Callewaere et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Chemokines play a key role in inflammation. They are expressed not only in neuroinflammatory conditions, but also constitutively by different cell types, including neurons in the normal brain, suggesting that they may act as modulators of neuronal functions. Here, we investigated a possible neuroendocrine role of the chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. We demonstrated the colocalization of SDF-1 and its receptor CXCR4 with arginine vasopressin (AVP) in the magnocellular neurons of the supraoptic nucleus (SON) and the paraventricular hypothalamic nucleus and on AVP projections to the neurohypophysis. Electrophysiological recordings of SON neurons demonstrated that SDF-1 affects the electrical activity of AVP neurons through CXCR4, resulting in changes in AVP release. We observed that SDF-1 can blunt the autoregulation of AVP release in vitro and counteract angiotensin II-induced plasma AVP release in vivo. Furthermore, a short-term physiological increase in AVP release induced by enhanced plasma osmolarity, which was produced by the administration of 1 M NaCl i.p., was similarly blocked by central injection of SDF-1 through CXCR4. A change in water balance by long-term salt loading induced a decrease in both SDF-1 and CXCR4 parallel to that of AVP immunostaining in SON. From these data, we demonstrate that chemokine actions in the brain are not restricted to inflammatory processes. We propose to add to the known autoregulation of AVP on its own neurons, a second autocrine system induced by SDF-1 able to modulate central AVP neuronal activity and release.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
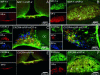
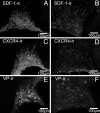
Fig. 1.
Colocalization of SDF-1 and CXCR4 receptor immunoreactivity (ir) with AVP (VP) in the hypothalamus and posterior pituitary in the rat. (A and B) Double immunohistochemistry of SDF-1 (stained in green) with AVP (stained in red) in the median eminence (A) and in the posterior pituitary (B). Simple stainings are presented in the small pictures of each panel. In the median eminence, SDF-1 strictly colocalizes with the AVP processes in the internal zone. In the pituitary, a strong SDF-1 immunostaining is observed in the posterior lobe of the pituitary, which is similar to the localization of AVP nerve terminals in this region, in particular in the probable Herring bodies (arrows). (Scale bars, 200 μm.) (C and D) Triple immunohistochemistry of CXCR4 (stained in green) with AVP (stained in red) and OT-expressing neurons (stained in blue) in the SON (C) and PVN (D). Eighty-five percent and 95% of the AVP neurons colocalize with CXCR4 immunoreactivity in the SON and PVN, respectively. No colocalization is observed between OT-positive neurons and CXCR4 immunostaining. Simple stainings are presented in the small pictures of each panel. (Scale bars, 100 μm.) (E and F) Double immunohistochemistry of CXCR4 (stained in green) with AVP (stained in red) in the median eminence (E) and in the posterior pituitary (F). Simple stainings are presented in the small pictures of each panel. (Scale bars, 200 μm.) In the median eminence, CXCR4 strictly colocalizes with the AVP processes in the internal zone. In the pituitary, a strong CXCR4 immunostaining is observed in the posterior lobe of the pituitary, which is similar to the localization of AVP nerve terminals in this region, in particular in the probable Herring bodies (arrows). All sections were observed with a fluorescence microscope (BX61; Olympus, Melville, NY), and images were computed for quantitative analysis.
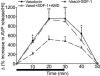
Fig. 2.
Electrophysiological characterization of the effect of SDF-1 on the action-potential firing pattern of AVP neurons in SON. Modulation by SDF-1 of action-potential firing in magnocellular neurosecretory cells is shown, as are typical normalized distribution histograms of the interspike intervals (ISIs) (left sides) of action potentials (right sides) recorded before, during, and after perfusion of 25 nM SDF-1 in different neurons. (A) SDF-1 inhibits the activity of AVP and decreases the duration of active periods. (B) SDF-1 stimulates the activity of AVP neurons and blunts the silent periods. (C) Sequence of a typical experimental protocol used to study the effect of SDF-1 and the implication of CXCR4. The number of action potentials per minute (AP/min) was recorded during 5-min periods under the conditions indicated. Note that the inhibitory effect of SDF-1 on action potentials is reproducible and reversibly inhibited by 10 μM AMD. Results are expressed as the means ± SEM. ∗∗, P < 0.01 vs. respective controls; §§, P < 0.01 vs. SDF-1.
Fig. 3.
Effect of SDF-1 on vasotocin-induced AVP release. Perifusion of hypothalamic tissues with 100 nM vasotocin for 30 min induces a 10-fold increase in AVP release. The effect is already observed after 10-min infusion with the peptide. SDF-1 (50 nM) significantly blunts vasotocin-induced AVP release. The inhibitory effect of SDF-1 is totally blocked by the CXCR4 antagonist AMD (10 μM). Results are expressed as Δ (% increase in AVP release per hypothalamus), mean ± SEM of three experiments. ∗, P < 0.05; and ∗∗, P < 0.01 vs. basal level.
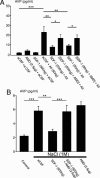
Fig. 4.
Effect of third-ventricle injection of SDF-1 on plasma AVP release induced by AII and NaCl. (A) Rats were injected in the third ventricle with artificial cerebrospinal fluid (aCSF) (control), AII, SDF-1, or AMD for 5 min. In the experimental groups in which two drugs were administered (or aCSF), the second was injected 7 min after the first. Plasma was collected 10 min after the final injection. Control rats received vehicle under similar experimental conditions. (B) Rats were injected with the various drugs in the third ventricle as described in A, and 4 min later they received an i.p. injection of 1 M NaCl. Control rats did not receive the NaCl injection. Plasma was collected 15 min after the final injection. SDF-1 blocks AVP release induced by AII or hypertonic solution, an effect counteracted by AMD. Results are expressed as the means ± SEM of at least six to eight animals per group. ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 vs. respective groups.
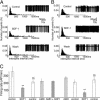
Fig. 5.
Effect of salt loading on SDF-1, CXCR4, and AVP (VP) immunoreactivity (ir) in the rat SON. SDF-1 colocalized with AVP in the control SON (15) dramatically decreases after salt loading (A and B). CXCR4 protein also decreases after 3% NaCl for 12 days (C and D) as does AVP (E and F). Both control (Left) and salt-loading (Right) pictures were taken at the same intensity. Similar data were obtained with the other animals tested. (Scale bars, 100 mm.)
Similar articles
- Cellular and subcellular evidence for neuronal interaction between the chemokine stromal cell-derived factor-1/CXCL 12 and vasopressin: regulation in the hypothalamo-neurohypophysial system of the Brattleboro rats.
Callewaere C, Fernette B, Raison D, Mechighel P, Burlet A, Calas A, Kitabgi P, Parsadaniantz SM, Rostène W. Callewaere C, et al. Endocrinology. 2008 Jan;149(1):310-9. doi: 10.1210/en.2007-1097. Epub 2007 Sep 27. Endocrinology. 2008. PMID: 17901225 Free PMC article. - Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons.
Banisadr G, Skrzydelski D, Kitabgi P, Rostène W, Parsadaniantz SM. Banisadr G, et al. Eur J Neurosci. 2003 Sep;18(6):1593-606. doi: 10.1046/j.1460-9568.2003.02893.x. Eur J Neurosci. 2003. PMID: 14511338 - A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia.
Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Höllt V, Schulz S. Stumm RK, et al. J Neurosci. 2002 Jul 15;22(14):5865-78. doi: 10.1523/JNEUROSCI.22-14-05865.2002. J Neurosci. 2002. PMID: 12122049 Free PMC article. - The CXCR4/SDF-1 chemokine axis: a potential therapeutic target for bone metastases?
Hirbe AC, Morgan EA, Weilbaecher KN. Hirbe AC, et al. Curr Pharm Des. 2010;16(11):1284-90. doi: 10.2174/138161210791034012. Curr Pharm Des. 2010. PMID: 20166978 Review. - CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion.
Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. Kucia M, et al. J Mol Histol. 2004 Mar;35(3):233-45. doi: 10.1023/b:hijo.0000032355.66152.b8. J Mol Histol. 2004. PMID: 15339043 Review.
Cited by
- Activation of the CXCR4 Receptor by Chemokine CXCL12 Increases the Excitability of Neurons in the Rat Central Amygdala.
Sowa JE, Tokarski K, Hess G. Sowa JE, et al. J Neuroimmune Pharmacol. 2024 Mar 2;19(1):9. doi: 10.1007/s11481-024-10112-2. J Neuroimmune Pharmacol. 2024. PMID: 38430337 - Sepsis-related stress response: known knowns, known unknowns, and unknown unknowns.
Peng J, Du B. Peng J, et al. Crit Care. 2010;14(4):179. doi: 10.1186/cc9103. Epub 2010 Jul 19. Crit Care. 2010. PMID: 20670385 Free PMC article. - A ligand-receptor interactome atlas of the zebrafish.
Chodkowski M, Zielezinski A, Anbalagan S. Chodkowski M, et al. iScience. 2023 Jul 12;26(8):107309. doi: 10.1016/j.isci.2023.107309. eCollection 2023 Aug 18. iScience. 2023. PMID: 37539027 Free PMC article. - GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4.
Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. Burbassi S, et al. J Neuroimmune Pharmacol. 2008 Mar;3(1):26-34. doi: 10.1007/s11481-007-9083-1. Epub 2007 Oct 25. J Neuroimmune Pharmacol. 2008. PMID: 18247130 Free PMC article. - Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus.
Heinisch S, Kirby LG. Heinisch S, et al. Neuroscience. 2009 Dec 15;164(3):1210-23. doi: 10.1016/j.neuroscience.2009.08.075. Epub 2009 Sep 10. Neuroscience. 2009. PMID: 19748551 Free PMC article.
References
- Brownstein M. J., Russell J. T., Gainer H. Science. 1980;207:373–378. - PubMed
- Rossi D., Zlotnik A. Annu. Rev. Immunol. 2000;18:217–242. - PubMed
- Banisadr G., Rostène W., Kitabgi P., Mélik Parsadaniantz S. Curr. Drug Targets Inflamm. Allergy. 2005;4:387–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous