A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons - PubMed (original) (raw)
A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons
Anthony M Rush et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Disease-producing mutations of ion channels are usually characterized as producing hyperexcitability or hypoexcitability. We show here that a single mutation can produce hyperexcitability in one neuronal cell type and hypoexcitability in another neuronal cell type. We studied the functional effects of a mutation of sodium channel Nav1.7 associated with a neuropathic pain syndrome, erythermalgia, within sensory and sympathetic ganglion neurons, two cell types where Nav1.7 is normally expressed. Although this mutation depolarizes resting membrane potential in both types of neurons, it renders sensory neurons hyperexcitable and sympathetic neurons hypoexcitable. The selective presence, in sensory but not sympathetic neurons, of the Nav1.8 channel, which remains available for activation at depolarized membrane potentials, is a major determinant of these opposing effects. These results provide a molecular basis for the sympathetic dysfunction that has been observed in erythermalgia. Moreover, these findings show that a single ion channel mutation can produce opposing phenotypes (hyperexcitability or hypoexcitability) in the different cell types in which the channel is expressed.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
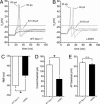
Fig. 1.
L858H renders DRG neurons hyperexcitable. (A and B) Action potentials were evoked from small (≤25 μm in diameter) DRG neurons by using depolarizing current injections from the RMP. _V_m, membrane potential. (A) Representative traces from a cell expressing WT Nav1.7 show subthreshold responses to 50- to 130-pA current injections and subsequent all-or-none action potentials evoked by injections of 135 pA (current threshold for this neuron) and 155 pA. (B) In contrast, in a cell expressing L858H, action potentials were evoked by a 60-pA current injection. The voltage for take-off of the all-or-none action potential (approximately −14.5 mV, dashed line) was similar for the neurons in A and B. (C) L858H causes a depolarizing shift in the RMP of DRG neurons. DRG neurons expressing WT Nav1.7 had an average RMP of −50.1 ± 0.9 mV (n = 20), whereas those expressing L858H mutant channels had a significantly (∗, P < 0.001) depolarized RMP of −44.9 ± 1.1 (_n_ = 25). (_D_) The average current threshold for action potential firing of DRG neurons expressing WT Nav1.7 channels was 120.6 ± 23.9 pA (_n_ = 20), whereas that of neurons expressing L858H mutant channels was significantly (∗, _P_ < 0.01) reduced to 69.2 ± 9.8 pA (_n_ = 25). (_E_) Action potential overshoot in cells expressing WT Nav1.7 channels (67.8 ± 3.0 mV, _n_ = 20) was not significantly different from that in cells expressing L858H mutant channels (64.4 ± 2.6 mV, _n_ = 20; _P_ > 0.05). The voltage of action potential take-off was unchanged (WT, −14.5 ± 1.2 mV, n = 20; L858H, −14.5 ± 1.3 mV, n = 25; P > 0.05). n.s., not significant.
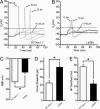
Fig. 2.
L858H renders SCG neurons hypoexcitable. (A and B) Action potentials were evoked by using depolarizing current injections from resting potential. (A) Representative traces from a cell expressing the WT channel show subthreshold responses to 15- to 20-pA current injections and subsequent all-or-none action potentials evoked by injections of ≥25 pA. (B) In contrast, in a cell expressing the L858H channel, action potentials required a ≥70-pA current injection. The voltage for take-off (dashed line) of the all-or-none action potential was unchanged. (C) L858H channels caused a depolarizing shift in the RMP of SCG neurons. SCG neurons expressing WT channels had an average RMP of −46.3 ± 0.8 mV (n = 15), whereas those expressing L858H had a significantly (P < 0.001) depolarized RMP of −41.6 ± 0.8 (_n_ = 17). (_D_) The average current threshold for action potential firing of SCG neurons expressing WT channels was 22.7 ± 3.6 pA (_n_ = 15), whereas that of neurons expressing L858H channels was significantly (∗, _P_ < 0.01) increased to 42.9 ± 6.3 pA (_n_ = 17). (_E_) Action potential overshoot in cells expressing WT channels (47.8 ± 3.4 mV, _n_ = 15) was significantly larger (∗, _P_ < 0.001) than that in cells expressing L858H (23.8 ± 4.7 mV, _n_ = 20). The voltage of action potential take-off was unchanged (WT, −23.1 ± 1.2 mV, _n_ = 15; L858H, −19.8 ± 1.3 mV, _n_ = 17; ∗, _P_ > 0.05).
Fig. 3.
The L858H mutation increases firing frequency in DRG and decreases firing frequency in SCG neurons. (A) Representative DRG neuron expressing WT Nav1.7 fires a single action potential in response to a 950-ms input of 100 pA from the RMP of this neuron (approximately −50 mV). (Inset) The same neuron fires multiple action potentials in response to a 250-pA stimulus. (B) Representative DRG neuron expressing L858H fires five action potentials in response to a 100-pA current injection from the RMP of this neuron (approximately −42 mV). (C) For the entire population of DRG neurons studied, the firing frequency evoked by 50-pA current stimuli was 0.32 ± 0.13 Hz after transfection with WT channels (n = 20) and 2.06 ± 0.79 Hz after transfection with L858H (n = 24; ∗, P < 0.05), and the firing frequency evoked by 100-pA stimuli was 0.89 ± 0.28 Hz after transfection with WT and 3.37 ± 1.13 Hz after transfection with L858H (∗, P < 0.05). (D) Representative SCG neuron expressing WT Nav1.7 fires six action potentials in response to a 950-ms input of 40 pA from the RMP (approximately −45 mV). (E) Representative SCG neuron expressing L858H fires only two action potentials in response to a 100-pA current injection from the RMP (approximately −40 mV). (Inset) When the cell was held at −60 mV to overcome the depolarization of the RMP caused by L858H, it produced four action potentials with an identical stimulus. (F) For the entire population of SCG neurons studied, the firing frequency evoked by 30-pA stimuli was 5.33 ± 1.5 Hz after transfection with WT channels (n = 14) and 0.63 ± 0.01 Hz after transfection with L858H channels (n = 15; P < 0.05). The firing frequency evoked by 40-pA stimuli was 7.05 ± 1.86 Hz after transfection with WT and 1.96 ± 1.0 Hz after transfection with L858H channels (∗, P < 0.05).
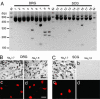
Fig. 4.
DRG neurons express Nav1.7 and Nav1.8; SCG neurons express Nav1.7 but not Nav1.8. (A) Restriction analysis of multiplex PCR amplification products from sodium channel domain 1 from adult DRG (lanes 1–9) and SCG (lanes 10–18). M, 100-bp ladder marker (Promega). Lanes 1 and 10 contain amplification products from DRG and SCG, respectively. Lanes 2–9 and 11–18 show results of cutting this DNA with EcoRV, EcoNI, AvaI, AccI, SphI, BamHI, AflII, and EcoRI, which are specific to subunits Nav1.1, Nav1.2, Nav1.3, Nav1.5/1.9, Nav1.6, Nav1.7/1.8, Nav1.8, and Nav1.9 (details can be found in Table 1, which is published as supporting information on the PNAS web site). Restriction products in lanes 2 and 5–9 show the presence of Nav1.1, Nav1.6, Nav1.7, Nav1.8, and Nav1.9 in DRG, in agreement with previous results (19). Restriction products in lanes 13, 15, and 16 show the presence of Nav1.3, Nav1.6, and Nav1.7 in SCG. (B and C) Immunostaining of Nav1.7 and Nav1.8 channels in DRG and SCG neurons in vivo and in cultured neurons. (B) Nav1.7 (a) and Nav1.8 (b) proteins are present in adult DRG neurons in vivo; Nav1.7 (c) and Nav1.8 (d) proteins are present in cultured DRG neurons from postnatal day 2 (P2) rat pups. (C) Nav1.7 (a), but not Nav1.8 (b), protein is present in adult SCG neurons in vivo; Nav1.7 (c), but not Nav1.8 (d), protein is present in cultured SCG neurons from P2 rat pups. (Scale bars, 50 μm.)
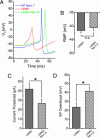
Fig. 5.
Coexpression of L858H and Nav1.8 channels rescues electrogenic properties in SCG neurons. When Nav1.8 was coexpressed with L858H, current threshold and action potential overshoot were restored, although the depolarization of the RMP induced by L858H persisted. (A) Suprathreshold action potentials recorded from representative SCG neurons transfected with WT (blue), L858H (red), and L858H plus Nav1.8 (green) channels. (B) Depolarized RMP in cells with L858H channels (−41.6 ± 0.76 mV, n = 17) was maintained with coexpression of Nav1.8 (−40.5 ± 1.01 mV, n = 17; P > 0.05). n.s., not significant. (C) Current threshold for action potential firing was reduced from 42.9 ± 6.3 pA (n = 17) for L858H to 26.8 ± 4.3 pA (n = 17) for L858H coexpressed with Nav1.8 (∗, P < 0.05). (D) Action potential overshoot in SCG neurons with L858H channel (23.8 ± 4.7 mV, n = 17) was increased when Nav1.8 was coexpressed with L858H (41.5 ± 4.6 mV, n = 17; ∗, P < 0.05).
Similar articles
- Differential effect of D623N variant and wild-type Na(v)1.7 sodium channels on resting potential and interspike membrane potential of dorsal root ganglion neurons.
Ahn HS, Vasylyev DV, Estacion M, Macala LJ, Shah P, Faber CG, Merkies IS, Dib-Hajj SD, Waxman SG. Ahn HS, et al. Brain Res. 2013 Sep 5;1529:165-77. doi: 10.1016/j.brainres.2013.07.005. Epub 2013 Jul 11. Brain Res. 2013. PMID: 23850641 - Paroxysmal extreme pain disorder M1627K mutation in human Nav1.7 renders DRG neurons hyperexcitable.
Dib-Hajj SD, Estacion M, Jarecki BW, Tyrrell L, Fischer TZ, Lawden M, Cummins TR, Waxman SG. Dib-Hajj SD, et al. Mol Pain. 2008 Sep 19;4:37. doi: 10.1186/1744-8069-4-37. Mol Pain. 2008. PMID: 18803825 Free PMC article. - A Nav1.7 channel mutation associated with hereditary erythromelalgia contributes to neuronal hyperexcitability and displays reduced lidocaine sensitivity.
Sheets PL, Jackson JO 2nd, Waxman SG, Dib-Hajj SD, Cummins TR. Sheets PL, et al. J Physiol. 2007 Jun 15;581(Pt 3):1019-31. doi: 10.1113/jphysiol.2006.127027. Epub 2007 Apr 12. J Physiol. 2007. PMID: 17430993 Free PMC article. - From genes to pain: Na v 1.7 and human pain disorders.
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Dib-Hajj SD, et al. Trends Neurosci. 2007 Nov;30(11):555-63. doi: 10.1016/j.tins.2007.08.004. Epub 2007 Oct 22. Trends Neurosci. 2007. PMID: 17950472 Review. - Painful peripheral neuropathy and sodium channel mutations.
Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG. Hoeijmakers JG, et al. Neurosci Lett. 2015 Jun 2;596:51-9. doi: 10.1016/j.neulet.2014.12.056. Epub 2014 Dec 31. Neurosci Lett. 2015. PMID: 25556685 Review.
Cited by
- The Domain II S4-S5 Linker in Nav1.9: A Missense Mutation Enhances Activation, Impairs Fast Inactivation, and Produces Human Painful Neuropathy.
Han C, Yang Y, de Greef BT, Hoeijmakers JG, Gerrits MM, Verhamme C, Qu J, Lauria G, Merkies IS, Faber CG, Dib-Hajj SD, Waxman SG. Han C, et al. Neuromolecular Med. 2015 Jun;17(2):158-69. doi: 10.1007/s12017-015-8347-9. Epub 2015 Mar 20. Neuromolecular Med. 2015. PMID: 25791876 - [Erythromelalgia: skin redness and pain].
Dusch M, Schmelz M. Dusch M, et al. Schmerz. 2019 Oct;33(5):475-490. doi: 10.1007/s00482-019-00401-8. Schmerz. 2019. PMID: 31485751 German. - Mutations at opposite ends of the DIII/S4-S5 linker of sodium channel Na V 1.7 produce distinct pain disorders.
Cheng X, Dib-Hajj SD, Tyrrell L, Wright DA, Fischer TZ, Waxman SG. Cheng X, et al. Mol Pain. 2010 Apr 29;6:24. doi: 10.1186/1744-8069-6-24. Mol Pain. 2010. PMID: 20429905 Free PMC article. - Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons.
Rush AM, Cummins TR, Waxman SG. Rush AM, et al. J Physiol. 2007 Feb 15;579(Pt 1):1-14. doi: 10.1113/jphysiol.2006.121483. Epub 2006 Dec 7. J Physiol. 2007. PMID: 17158175 Free PMC article. Review. - Mutation I136V alters electrophysiological properties of the Na(v)1.7 channel in a family with onset of erythromelalgia in the second decade.
Cheng X, Dib-Hajj SD, Tyrrell L, Waxman SG. Cheng X, et al. Mol Pain. 2008 Jan 2;4:1. doi: 10.1186/1744-8069-4-1. Mol Pain. 2008. PMID: 18171466 Free PMC article.
References
- Waxman S. G., Dib-Hajj S. Trends Mol. Med. 2005;11:555–562. - PubMed
- Dib-Hajj S. D., Rush A. M., Cummins T. R., Hisama F. M., Novella S., Tyrrell L., Marshall L., Waxman S. G. Brain. 2005;128:1847–1854. - PubMed
- Drenth J. P., Te Morsche R. H., Guillet G., Taieb A., Kirby R. L., Jansen J. B. J. Invest. Dermatol. 2005;124:1333–1338. - PubMed
- Michiels J. J., te Morsche R. H., Jansen J. B., Drenth J. P. Arch. Neurol. (Chicago) 2005;62:1587–1590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases