The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation - PubMed (original) (raw)
The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation
Tobias Ragoczy et al. Genes Dev. 2006.
Abstract
We have examined the relationship between nuclear localization and transcriptional activity of the endogenous murine beta-globin locus during erythroid differentiation. Murine fetal liver cells were separated into distinct erythroid maturation stages by fluorescence-activated cell sorting, and the nuclear position of the locus was determined at each stage. We find that the beta-globin locus progressively moves away from the nuclear periphery with increasing maturation. Contrary to the prevailing notion that the nuclear periphery is a repressive compartment in mammalian cells, beta(major)-globin expression begins at the nuclear periphery prior to relocalization. However, relocation of the locus to the nuclear interior with maturation is accompanied by an increase in beta(major)-globin transcription. The distribution of nuclear polymerase II (Pol II) foci also changes with erythroid differentiation: Transcription factories decrease in number and contract toward the nuclear interior. Moreover, both efficient relocalization of the beta-globin locus from the periphery and its association with hyperphosphorylated Pol II transcription factories require the locus control region (LCR). These results suggest that the LCR-dependent association of the beta-globin locus with transcriptionally engaged Pol II foci provides the driving force for relocalization of the locus toward the nuclear interior during erythroid maturation.
Figures
Figure 1.
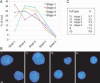
Isolation of erythroid cells at progressive maturation stages. (A) FACS profiles of the fetal liver cells: pairwise comparison of ERY-and 1 CD117 staining (panel i), Ter-119 and CD117 (panel ii), and ERY-1 and Ter-119 (panel iii). The arrows indicate changes in the fluorescence observed with increasing erythroid maturation. (B) Wright-Giemsa staining of sorted fetal liver populations. The four fractions (stage 1: CD117+, ERY-1−, TER-119−; stage 2: CD117+, ERY-1+, TER-119−; stage 3: CD117−, ERY-1+ TER-119+; stage 4: CD117−, ERY-1−, TER-119+) exhibit a progressive decrease in cell size, increase in cytoplasm to nuclear volume ratio, nuclear compaction, and enucleation.
Figure 2.
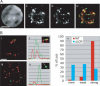
Nuclear location of the β-globin locus. (A) Distribution of the β-globin loci among five concentric shells at the different maturation stages as assayed by DNA FISH (each shell encompasses 20% of the nuclear radius, shell 1 representing the periphery and shell 5 the center of the nucleus). Image stacks of formaldehyde-fixed cells were collapsed onto a single plane and analyzed in 2D. Between 150 and 300 loci were scored for each cell population. (B, panels i_–_iv) Examples of β-globin locus position at the sorted maturation stages 1–4 by DNA FISH. The β-globin locus probe is stained green; nuclear DNA is counterstained blue with DAPI. Bars, 2 μm. (C) Association of the β-globin locus with PCH by DNA FISH. Nuclear volumes were reconstructed and rotated in 3D, and colocalization of the β-globin locus probe with γ-satellite repeat probe scored. All four fetal liver stages and splenic B cells and ES cells were analyzed.
Figure 3.
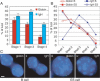
Location of the β-globin and IgH loci is gene and tissue specific. (A) The nuclear position of the IgH and β-globin loci was compared at three different erythroid maturation stages by DNA FISH. Shown are only the frequencies of peripheral loci within 20% of the nuclear radius from the envelope (red bars indicate β-globin, blue bars indicate IgH locus, and error bars indicate SE). (B) Comparison of the nuclear positions of the β-globin and IgH loci in B and ES cells. The graph shows the distribution of the gene loci among five concentric shells in B- and ES-cell nuclei (each shell encompasses 20% of the nuclear radius, shell 1 representing the periphery and shell 5 the center of the nucleus; red open squares indicate _β_-globin locus in B cells, red closed squares indicate β-globin locus in ES cells, blue closed circles indicate IgH locus in B cells, and blue open circles indicate IgH locus in ES cells). (C) Examples of β-globin and IgH locus position in B and ES cells by DNA FISH. The locus probes are green; nuclear DNA is counterstained blue with DAPI. Bars, 2 μm.
Figure 4.
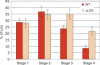
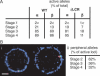
The LCR is required for relocation away from the periphery. The nuclear position of the ΔLCR globin locus during erythroid differentiation was compared with the wild-type allele by DNA FISH. Shown are only the frequencies of peripheral loci (within 20% of the nuclear radius from the envelope; red bars indicate wild type, orange bars indicate ΔLCR locus, and error bars indicate SE).
Figure 5.
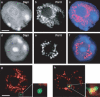
Nuclear distribution of Pol II during erythroid differentiation and position of the β-globin locus with respect to Pol II. Sorted fetal liver cells (a_–_c, stage 1; d_–_f, stage 4) were immunostained for Pol II. (a,d) Nuclear counterstain DAPI. (b,e) Pol II. (c,f) Merged images of DAPI (blue) and Pol II (red). (g) In immature erythroid cells (stage 1), the predominantly peripheral _β_-globin locus (green) falls just outside Pol II staining (red). (h) In mature cells (stage 4), the locus lies most often in the nuclear interior and overlaps with strong foci of Pol II staining. Bars, 2 μm.
Figure 6.
The LCR is required for association with hyperphosphorylated Pol II. (A) Pol II hyperphosphorylated at the CTD (Pol II-Ser5-P) stains a subpopulation of Pol II foci. (Panel i) DAPI stain of a mature fetal liver cell. (Panel ii) Pol II stain. (Panel iii) Pol II-Ser5-P. (Panel iv) Merged image of panels ii and iii (Pol II, red; Pol II-Ser5-P, green). Bar, 2 μm. (B, panel i) In mature erythroid cells (stage 4), the wild-type β-globin locus (green) consistently colocalizes with Pol II-Ser5-P (red), unlike the ΔLCR alleles (in panel iii). Bar, 2 μm. (Panels ii,iv) Linescans of the images on the left, demonstrating the signal overlap between the β-globin locus and Pol II-Ser5-P for the wild-type locus (panel ii) and lack thereof for the mutant (panel iv). (Panel v) Frequency of colocalization of wild-type (red) and ΔLCR (blue) globin loci with Pol II-Ser5-P foci. “Weak” foci exhibit a signal intensity of <20% of the brightest foci detectable in a given nucleus.
Figure 7.
Expression of _α_- and _β_-globin during erythroid differentiation by primary transcript RNA FISH. (A) Sorted fetal liver cell populations were fixed and probed with oligonucleotides to introns of α and β-major transcripts. Seventy to 100 nuclei were analyzed for each cell fraction. (B) β-globin expression at the nuclear periphery. (Panel i) Examples of transcriptionally active peripheral β-globin loci. Fetal liver cells were probed for the β-globin locus (green) and β-major primary transcripts (red) by RNA/DNA FISH followed by immunostaining of lamin B (blue). Bar, 2 μm. Especially at early maturation stages, the β-globin locus colocalizes with the nuclear lamina, even when the locus is transcriptionally active. (Panel ii) Percentage of active β-globin loci at the nuclear periphery by 3D analysis at all three erythroid maturation stages that exhibit globin transcription. At least 50–60 active loci were analyzed for each stage.
Comment in
- Constricting restricted transcription: the (actively?) shrinking web.
Fraser P, Engel JD. Fraser P, et al. Genes Dev. 2006 Jun 1;20(11):1379-83. doi: 10.1101/gad.1438106. Genes Dev. 2006. PMID: 16751176 No abstract available.
Similar articles
- The beta -globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation.
Sawado T, Halow J, Bender MA, Groudine M. Sawado T, et al. Genes Dev. 2003 Apr 15;17(8):1009-18. doi: 10.1101/gad.1072303. Epub 2003 Apr 2. Genes Dev. 2003. PMID: 12672691 Free PMC article. - The hypersensitive sites of the murine β-globin locus control region act independently to affect nuclear localization and transcriptional elongation.
Bender MA, Ragoczy T, Lee J, Byron R, Telling A, Dean A, Groudine M. Bender MA, et al. Blood. 2012 Apr 19;119(16):3820-7. doi: 10.1182/blood-2011-09-380485. Epub 2012 Feb 29. Blood. 2012. PMID: 22378846 Free PMC article. - Subcellular transport of EKLF and switch-on of murine adult beta maj globin gene transcription.
Shyu YC, Lee TL, Wen SC, Chen H, Hsiao WY, Chen X, Hwang J, Shen CK. Shyu YC, et al. Mol Cell Biol. 2007 Mar;27(6):2309-23. doi: 10.1128/MCB.01875-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242208 Free PMC article. - Sumoylation of p45/NF-E2: nuclear positioning and transcriptional activation of the mammalian beta-like globin gene locus.
Shyu YC, Lee TL, Ting CY, Wen SC, Hsieh LJ, Li YC, Hwang JL, Lin CC, Shen CK. Shyu YC, et al. Mol Cell Biol. 2005 Dec;25(23):10365-78. doi: 10.1128/MCB.25.23.10365-10378.2005. Mol Cell Biol. 2005. PMID: 16287851 Free PMC article. - Chromatin loop formation in the β-globin locus and its role in globin gene transcription.
Kim A, Dean A. Kim A, et al. Mol Cells. 2012 Jul;34(1):1-5. doi: 10.1007/s10059-012-0048-8. Epub 2012 May 18. Mol Cells. 2012. PMID: 22610406 Free PMC article. Review.
Cited by
- The Hematopoietic Stem and Progenitor Cell Cistrome: GATA Factor-Dependent cis-Regulatory Mechanisms.
Hewitt KJ, Johnson KD, Gao X, Keles S, Bresnick EH. Hewitt KJ, et al. Curr Top Dev Biol. 2016;118:45-76. doi: 10.1016/bs.ctdb.2016.01.002. Epub 2016 Feb 26. Curr Top Dev Biol. 2016. PMID: 27137654 Free PMC article. Review. - Regulating a master regulator: establishing tissue-specific gene expression in skeletal muscle.
Aziz A, Liu QC, Dilworth FJ. Aziz A, et al. Epigenetics. 2010 Nov-Dec;5(8):691-5. doi: 10.4161/epi.5.8.13045. Epub 2010 Nov 1. Epigenetics. 2010. PMID: 20716948 Free PMC article. Review. - The genome organization of Neurospora crassa at high resolution uncovers principles of fungal chromosome topology.
Rodriguez S, Ward A, Reckard AT, Shtanko Y, Hull-Crew C, Klocko AD. Rodriguez S, et al. G3 (Bethesda). 2022 May 6;12(5):jkac053. doi: 10.1093/g3journal/jkac053. G3 (Bethesda). 2022. PMID: 35244156 Free PMC article. - Dissecting the function of the adult β-globin downstream promoter region using an artificial zinc finger DNA-binding domain.
Barrow JJ, Li Y, Hossain M, Huang S, Bungert J. Barrow JJ, et al. Nucleic Acids Res. 2014 Apr;42(7):4363-74. doi: 10.1093/nar/gku107. Epub 2014 Feb 4. Nucleic Acids Res. 2014. PMID: 24497190 Free PMC article. - Differentiation-dependent chromosomal organization changes in normal myogenic cells are absent in rhabdomyosarcoma cells.
Ibarra J, Hershenhouse T, Almassalha L, MacQuarrie KL. Ibarra J, et al. bioRxiv [Preprint]. 2023 May 11:2023.05.11.540394. doi: 10.1101/2023.05.11.540394. bioRxiv. 2023. PMID: 37214969 Free PMC article. Updated. Preprint.
References
- Andrulis E.D., Neiman A.M., Zappulla D.C., Sternglanz R. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature. 1998;394:592–595. - PubMed
- Bacon E.R., Sytkowski A.J. Identification and characterization of a differentiation-specific antigen on normal and malignant murine erythroid cells. Blood. 1987;69:103–108. - PubMed
- Bender M.A., Bulger M., Close J., Groudine M. β-Globin gene switching and DNase I sensitivity of the endogenous β-globin locus in mice do not require the locus control region. Mol. Cell. 2000;5:387–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources