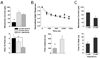The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells - PubMed (original) (raw)
Comparative Study
The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells
Erin J Warren et al. Eur J Neurosci. 2006 May.
Abstract
In mammals, the master circadian clock resides in the suprachiasmatic nuclei (SCN) of the hypothalamus. The period and phase of the circadian pacemaker are calibrated by direct photic input from retinal ganglion cells (RGCs). SCN-projecting RGCs respond to light in the absence of rod- and cone-driven synaptic input, a property for which they are termed intrinsically photosensitive. In SCN-projecting RGCs, light activates a nonselective cationic current that displays inward and outward rectification. The goal of the present study was to investigate the identity of the light-activated ion channel and the intracellular signaling pathway leading to its activation. We considered two candidate channels, cyclic nucleotide-gated (CNG) channels and transient receptor potential (TRP) channels, which mediate vertebrate and invertebrate phototransduction, respectively. We report that the intrinsic light response relies upon a G-protein-dependent process. Although our data indicate that cyclic nucleotides modulate the signaling pathway, CNG channels do not appear to conduct the light-activated current because (i) cyclic nucleotides in the pipette solution do not activate a conductance or completely block the light response, (ii) CNG channel blockers fail to inhibit the light response, (iii) the effects of internal and external divalent cations are inconsistent with their effects on CNG channels, and (iv) immunohistochemistry reveals no CNG channels in SCN-projecting RGCs. Finally, we show that the pharmacology of the light-activated channel resembles that of some TRPC channel family members; the response is blocked by lanthanides and ruthenium red and SK&F 96365, and is enhanced by flufenamic acid and 1-oleoyl-2-acetyl-sn-glycerol. Furthermore, immunohistochemical experiments reveal that TRPC6 is expressed in many RGCs, including those that express melanopsin.
Figures
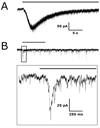
FIG. 1
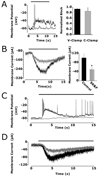
Intrinsic and synaptically driven light responses from RGCs held at −60 mV. (A) Whole-cell voltage-clamp recording of an intrinsic light response recorded from an SCN-projecting RGC. (B) Upper panel depicts a synaptic light response from a non-SCN-projecting Type I RGC plotted on the same time scale as the trace in A. The box is expanded in the lower panel to show an increase in synaptic currents following the onset of light. Black bars represent light stimulation.
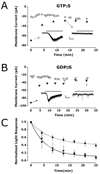
FIG. 2
The intrinsic light response is G-protein dependent. Whole-cell baseline currents were sampled throughout the recordings (○). Triangles represent the peak of the light-activated currents. Inset traces show light responses before (left) and after (right) block of response. Scale bars: 2 s, 20 pA; bar represents light stimulation. (A) In one SCN-projecting RGC, 300 μm GTPγS (▲) caused a 55% reduction in the amplitude of the light response between the first light stimulus and a second light stimulus delivered 4 min later. The amplitudes of responses elicited by subsequent steps of light were reduced by 91%. (B) In one SCN-projecting RGC, 1 mm GDPβS (▼) in the recording pipette reduced the light response by 96%. (C) Normalized group data from seven control cells containing 300 μm GTP (■) is plotted alongside normalized data from cells containing GTPγS (▲; n = 4) and GDPβS (▼; n = 4).
FIG. 3
Cyclic nucleotides modulate the light response but do not gate the light-activated channel. (A) Average whole-cell baseline currents were plotted from cells with pipette solutions containing either 500 μM cAMP (●) or 500 μM cGMP (∆). One-way anova detected no significant change in baseline currents of either population over the 33 min recording period. (B and C) Baseline currents (○) and peak light-activated currents (▲) from two SCN-projecting RGCs with pipette solutions containing either 500 μM cAMP (B) or 500 μM cGMP (C): 500 μM IBMX application is represented by hatched box. (D) Coapplication of IBMX with cAMP or with cGMP significantly reduced the light response. *Significantly different from control (P < 0.002 with cAMP and P < 0.012 with cGMP).
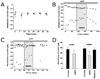
FIG. 4
Divalent ions modulate the light-activated current. (A) Upper: the average light response amplitude is significantly smaller when 18 mM BAPTA (black) is included in the pipette compared with 10 mM EGTA (hatched) and 0.1 mM (grey). Lower: the rate of the response is significantly longer in 18 mm BAPTA than in 0.1 mM EGTA. (B) Upper: light responses were normalized to the first maximal light response and the averages for each group plotted over 1600 s. Both cells with 0.1 mM EGTA (●) and cells with 10 mM EGTA (■) in the pipette solution reached a steady-state light response amplitude around 50% of initial amplitude. Lower: the time constants of rundown were calculated by fitting data from each cell to a single exponential decay and averaging within a group. (C) Upper: increasing internal magnesium from 1 mM to 4 mM reduced the amplitude of the light response. Lower: the increase in magnesium also slowed the development of the lightactivated inward current. Neither of these findings were significant by Student's _t_-test. Asterisks indicate: P < 0.004 compared with 10 mM EGTA in A upper panel; P < 0.025 in A lower panel; P = 0.05 in panel B.
FIG. 5
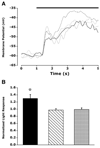
TRP channel antagonists block the intrinsic light response. (A) Left: 20 μM ruthenium red blocked the intrinsic light response in an SCN-projecting RGC during a perforated-patch current clamp recording. Right: summary data showing percentage block by ruthenium red in three whole-cell voltage-clamp (black) and three perforated-patch current-clamp recordings (grey). (B) SK&F (200 μM), a TRPC channel blocker, significantly reduced the amplitude of the light-activated current by 48.4% ± 22 (n = 8). (C) LaCl3 (100 μM) blocked the intrinsic light response in a perforated-patch current-clamp recording. (D) GdCl3 (200 μM) reduced the whole-cell lightactivated current in this SCN-projecting RGC by 57%. Black traces in A–D represent control data and light stimulation is represented by black bars. *P < 0.005.
FIG. 6
1-Oleoyl-2-acetyl-_sn_-glycerol (OAG) potentiated the intrinsic light response in a PKC-dependent manner. (A) Data taken from one SCN-projecting neuron, low-pass filtered at 2 Hz. In order of applications: control, black; 20 μM sphingosine, dotted; 100 μM OAG, dashed; OAG and sphingosine, grey. (B) OAG (100 μM) significantly potentiated the intrinsic light response in 18 SCN-projecting RGCs during perforated-patch current-clamp recordings (black). The PKC antagonist sphingosine (20 μM) had no effect on the light response when applied alone to five cells (hatched). Coapplication of OAG and sphingosine blocked the OAG-induced potentiation (n = 4, grey). *P = 0.008.
FIG. 7
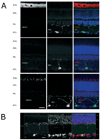
Ion channel expression in melanopsin-containing RGCs. (A) CNG channels do not colocalize with melanopsin. Retinal sections were immunostained for melanopsin (green) and CNGA1 (top, red), CNGA2 (middle, red) and CNGA3 (bottom, red). DAPI-staining of cell nuclei (blue) was used to visualize retinal layers. (B) TRPC6 channels are found in most RGCs, including those that express melanopsin. Melanopsin (green) was visualized by tyramide signal amplification, and TRPC6 (red) was visualized using conventional immunostaining techniques (see Methods). Scale bars, 20 μM. Abbreviations: POS (photoreceptor outer segments); ONL (outer nuclear layer); OPL (outer plexiform layer); INL (inner nuclear layer); IPL (inner plexiform layer); GCL (ganglion cell layer).
Similar articles
- M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina.
Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK, Zhang DQ. Prigge CL, et al. J Neurosci. 2016 Jul 6;36(27):7184-97. doi: 10.1523/JNEUROSCI.3500-15.2016. J Neurosci. 2016. PMID: 27383593 Free PMC article. - Intrinsic light responses of retinal ganglion cells projecting to the circadian system.
Warren EJ, Allen CN, Brown RL, Robinson DW. Warren EJ, et al. Eur J Neurosci. 2003 May;17(9):1727-35. doi: 10.1046/j.1460-9568.2003.02594.x. Eur J Neurosci. 2003. PMID: 12752771 Free PMC article. - Synaptic inputs to retinal ganglion cells that set the circadian clock.
Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Brown RL. Perez-Leon JA, et al. Eur J Neurosci. 2006 Aug;24(4):1117-23. doi: 10.1111/j.1460-9568.2006.04999.x. Eur J Neurosci. 2006. PMID: 16930437 Free PMC article. - Melanopsin--shedding light on the elusive circadian photopigment.
Brown RL, Robinson PR. Brown RL, et al. Chronobiol Int. 2004 Mar;21(2):189-204. doi: 10.1081/cbi-120037816. Chronobiol Int. 2004. PMID: 15332341 Free PMC article. Review. - Intrinsically photosensitive retinal ganglion cells.
Pickard GE, Sollars PJ. Pickard GE, et al. Sci China Life Sci. 2010 Jan;53(1):58-67. doi: 10.1007/s11427-010-0024-5. Epub 2010 Feb 12. Sci China Life Sci. 2010. PMID: 20596956 Review.
Cited by
- M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina.
Prigge CL, Yeh PT, Liou NF, Lee CC, You SF, Liu LL, McNeill DS, Chew KS, Hattar S, Chen SK, Zhang DQ. Prigge CL, et al. J Neurosci. 2016 Jul 6;36(27):7184-97. doi: 10.1523/JNEUROSCI.3500-15.2016. J Neurosci. 2016. PMID: 27383593 Free PMC article. - Non-selective cation channels, transient receptor potential channels and ischemic stroke.
Simard JM, Tarasov KV, Gerzanich V. Simard JM, et al. Biochim Biophys Acta. 2007 Aug;1772(8):947-57. doi: 10.1016/j.bbadis.2007.03.004. Epub 2007 Mar 19. Biochim Biophys Acta. 2007. PMID: 17446049 Free PMC article. Review. - Vertebrate vision: TRP channels in the spotlight.
Ribelayga C. Ribelayga C. Curr Biol. 2010 Mar 23;20(6):R278-80. doi: 10.1016/j.cub.2010.02.012. Curr Biol. 2010. PMID: 20334836 Free PMC article. - Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development.
Schmidt TM, Taniguchi K, Kofuji P. Schmidt TM, et al. J Neurophysiol. 2008 Jul;100(1):371-84. doi: 10.1152/jn.00062.2008. Epub 2008 May 14. J Neurophysiol. 2008. PMID: 18480363 Free PMC article. - Clinical implications of the melanopsin-based non-image-forming visual system.
Ksendzovsky A, Pomeraniec IJ, Zaghloul KA, Provencio JJ, Provencio I. Ksendzovsky A, et al. Neurology. 2017 Mar 28;88(13):1282-1290. doi: 10.1212/WNL.0000000000003761. Epub 2017 Mar 1. Neurology. 2017. PMID: 28251921 Free PMC article. Review.
References
- Bandyopadhyay BC, Payne R. Variants of TRP ion channel mRNA present in horseshoe crab ventral eye and brain. J. Neurochem. 2004;91:825–835. - PubMed
- Baylor DA. Photoreceptor signals and vision Proctor lecture. Invest. Ophthalmol. Vis. Sci. 1987;28:34–49. - PubMed
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 1997;272:29672–29680. - PubMed
- Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr. Opin. Neurobiol. 2005;15:343–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources