Role of dopamine in the primate caudate nucleus in reward modulation of saccades - PubMed (original) (raw)
Role of dopamine in the primate caudate nucleus in reward modulation of saccades
Kae Nakamura et al. J Neurosci. 2006.
Abstract
Expected reward impacts behavior and neuronal activity in brain areas involved in sensorimotor processes. However, where and how reward signals affect sensorimotor signals is unclear. Here, we show evidence that reward-dependent modulation of behavior depends on normal dopamine transmission in the striatum. Monkeys performed a visually guided saccade task in which expected reward gain was different depending on the position of the target. Saccadic reaction times were reliably shorter on large-reward trials than on small-reward trials. When position-reward contingency was switched, the reaction time difference changed rapidly. Injecting dopamine D1 antagonist into the caudate significantly attenuated the reward-dependent saccadic reaction time changes. Conversely, injecting D2 antagonist into the same region enhanced the reward-dependent changes. These results suggest that reward-dependent changes in saccadic eye movements depend partly on dopaminergic modulation of neuronal activity in the caudate nucleus.
Figures
Figure 1.
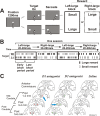
Visually guided saccade task with position-dependent reward difference. The white arrows indicate the direction of the gaze. A, After 1200 ms of fixation on the central fixation point, the target came on either on the left or right, and the monkey had to make a saccade to the target. In left-large condition, left saccades were followed by large reward, whereas right saccades were followed by small reward; in right-large condition, the position–reward contingency was reversed. B, Left-large and right-large conditions were alternated every 20–28 trials as blocks. The location of the target was determined pseudo-randomly. For later analysis, trials within a block were divided into early and later block periods. One session of experiment consists of four to six blocks. Rt, Right; Lt, left. C, Injection sites of D1 antagonist (SCH23390), D2 antagonist (Eticlopride), and saline are mapped on coronal magnetic resonance images posterior to the anterior commissure with 2 mm intervals (monkey S). Based on the drug effects, we divided the injection sites into two parts: midcaudate, 1–4 mm posterior to the anterior commissure and postcaudate, 5–6 mm posterior to the anterior commissure. The arrows indicate the examples shown in Figures 2, A and B (for D1 antagonist), and 5, A and B (for D2 antagonist).
Figure 2.
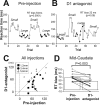
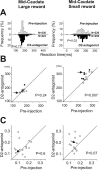
Reward-dependent reaction time bias was attenuated by D1 antagonist injection. A, B, An example of D1 antagonist injection experiment with data collected before (A) and after (B) the injection. The injection was made in the right, midcaudate (indicated in Fig. 1_C_). Reaction times for leftward saccades (contralateral to the injection site) are plotted against the trial number. The leftward saccades were followed by small reward in one block of trials (open circles) and large reward in another block (filled circles). The data points are not regularly spaced because the order of leftward and rightward (data not shown) saccades was pseudorandom. The difference in the mean saccade reaction times between large- and small-reward trials was computed as the reaction time bias (RT bias) and the p value of Mann–Whitney U test (see Materials and Methods). C, Reaction time biases before and after individual D1 antagonist injections (n = 21) for contralateral saccades are plotted in abscissa and ordinate, respectively. Different symbols indicate the distance of injection sites from the anterior commissure: 1–2 mm (filled squares), 3–4 mm (filled circles), 5–6 mm (open triangles). The anterior injection sites (1–4 mm) and the posterior sites (5–6 mm) were designated as the midcaudate and the postcaudate, respectively (see Results). D, Changes in the reaction time bias from preinjection control to D1 antagonist injection to midcaudate (n = 12). The lines show the data of individual experiments; solid and dotted lines indicate the data from monkey S and monkey L, respectively. Statistical difference in data between control and test is shown by the p value of Wilcoxon signed rank test. Error bars indicate 1 SE. The arrow in C and the thick line in D indicate the data presented in A and B.
Figure 3.
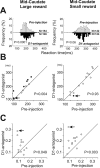
Changes in saccade reaction times by D1 antagonist injection in the midcaudate on large-reward trials (left column) and on small-reward trials (right column). A, The distributions of reaction times on all trials obtained from eight experiments without a gap between fixation offset and target onset. In each panel, the top (white bars) and bottom (black bars) histograms indicate the preinjection and postinjection sessions, respectively. B, Mean reaction times for individual experiments. Filled circles indicate significant differences between the preinjection and postinjection sessions (Mann–Whitney U test, p < 0.05); open circles indicate nonsignificant differences. C, Coefficient of variation of reaction times. The arrows in B and C indicate the data presented in Figure 2, A and B.
Figure 4.
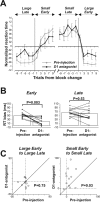
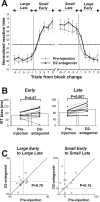
The effects of D1 antagonist were stronger during the earlier period of block after the reversal of position–reward contingency. A, Trial-by-trial changes in reaction times for contralateral saccades. The preinjection data are shown in gray; the postinjection data are in black. Normalized reaction times (see Materials and Methods) averaged across all 12 experiments with injections in the midcaudate are plotted against the number of trials before and after the time when the block (position–reward contingency) was changed. Data for adjacent two trials are averaged: trial number 1 indicates the average of the data for trial 1 and 2; trial number 3 indicates the average for trial 3 and 4. B, The change in the reaction time (RT) bias for contralateral saccades during the early (left) and late (right) periods of block. The format is the same as in Figure 2_D_. C, Changes in the mean reaction times from the early to late period of the block, shown separately for the large-reward block (left) and the small-reward block (right). The data were obtained for contralateral saccades before (abscissa) and after (ordinate) D1 antagonist injections in the midcaudate (n = 12). Error bars indicate 1 SE.
Figure 5.
Reward-dependent reaction time (RT) bias was enhanced by D2 antagonist injection. The same format as Figure 2.
Figure 6.
Changes in saccade reaction times by D2 antagonist injection in the midcaudate for large-reward trials (left column) and small-reward trials (right column). The same format as Figure 3.
Figure 7.
The effects of D2 antagonist were stronger some trials after the reversal of position–reward contingency. The same format as Figure 4. RT, Reaction time. Error bars indicate 1 SE.
Figure 8.
Summary of the effects of dopamine D1 and D2 antagonist injections to the midcaudate. The direction of arrows indicates an increase (upward) or a decrease (downward) of the values. NS, Statistically not significant; Contra, contralateral; Ipsi, ipsilateral.
Similar articles
- Basal ganglia mechanisms of reward-oriented eye movement.
Hikosaka O. Hikosaka O. Ann N Y Acad Sci. 2007 May;1104:229-49. doi: 10.1196/annals.1390.012. Epub 2007 Mar 14. Ann N Y Acad Sci. 2007. PMID: 17360800 Review. - Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus.
Watanabe K, Lauwereyns J, Hikosaka O. Watanabe K, et al. J Neurosci. 2003 Nov 5;23(31):10052-7. doi: 10.1523/JNEUROSCI.23-31-10052.2003. J Neurosci. 2003. PMID: 14602819 Free PMC article. - Eye movements in monkeys with local dopamine depletion in the caudate nucleus. I. Deficits in spontaneous saccades.
Kato M, Miyashita N, Hikosaka O, Matsumura M, Usui S, Kori A. Kato M, et al. J Neurosci. 1995 Jan;15(1 Pt 2):912-27. doi: 10.1523/JNEUROSCI.15-01-00912.1995. J Neurosci. 1995. PMID: 7823189 Free PMC article. - Psychopharmacology of conditioned reward: evidence for a rewarding signal at D1-like dopamine receptors.
Sutton MA, Beninger RJ. Sutton MA, et al. Psychopharmacology (Berl). 1999 May;144(2):95-110. doi: 10.1007/s002130050982. Psychopharmacology (Berl). 1999. PMID: 10394990 Review.
Cited by
- Dopamine neurons projecting to the posterior striatum reinforce avoidance of threatening stimuli.
Menegas W, Akiti K, Amo R, Uchida N, Watabe-Uchida M. Menegas W, et al. Nat Neurosci. 2018 Oct;21(10):1421-1430. doi: 10.1038/s41593-018-0222-1. Epub 2018 Sep 3. Nat Neurosci. 2018. PMID: 30177795 Free PMC article. - Central serotonin modulates neural responses to virtual violent actions in emotion regulation networks.
Wolf D, Klasen M, Eisner P, Zepf FD, Zvyagintsev M, Palomero-Gallagher N, Weber R, Eisert A, Mathiak K. Wolf D, et al. Brain Struct Funct. 2018 Sep;223(7):3327-3345. doi: 10.1007/s00429-018-1693-2. Epub 2018 Jun 8. Brain Struct Funct. 2018. PMID: 29948188 Free PMC article. - Frontal eye field and caudate neurons make different contributions to reward-biased perceptual decisions.
Fan Y, Gold JI, Ding L. Fan Y, et al. Elife. 2020 Nov 27;9:e60535. doi: 10.7554/eLife.60535. Elife. 2020. PMID: 33245044 Free PMC article. - Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors.
Darvas M, Palmiter RD. Darvas M, et al. Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14664-9. doi: 10.1073/pnas.0907299106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667174 Free PMC article. - Conditional Regulation of Blood Pressure in Response to Emotional Stimuli by the Central Nucleus of the Amygdala in Rats.
Yamanaka K, Waki H. Yamanaka K, et al. Front Physiol. 2022 Jun 1;13:820112. doi: 10.3389/fphys.2022.820112. eCollection 2022. Front Physiol. 2022. PMID: 35721563 Free PMC article.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A (2000). Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3:226–230. - PubMed
- Amador N, Schlag-Rey M, Schlag J (2000). Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol 84:2166–2170. - PubMed
- Bari AA, Pierce RC (2005). D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 135:959–968. - PubMed
- Beninger RJ, Miller R (1998). Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev 22:335–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources