Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury - PubMed (original) (raw)
. 2006 Jun;116(6):1606-14.
doi: 10.1172/JCI27183. Epub 2006 May 18.
Affiliations
- PMID: 16710477
- PMCID: PMC1462943
- DOI: 10.1172/JCI27183
Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury
R Gisli Jenkins et al. J Clin Invest. 2006 Jun.
Abstract
Activation of latent TGF-beta by the alpha(v)beta6 integrin is a critical step in the development of acute lung injury. However, the mechanism by which alpha(v)beta6-mediated TGF-beta activation is regulated has not been identified. We show that thrombin, and other agonists of protease-activated receptor 1 (PAR1), activate TGF-beta in an alpha(v)beta6 integrin-specific manner. This effect is PAR1 specific and is mediated by RhoA and Rho kinase. Intratracheal instillation of the PAR1-specific peptide TFLLRN increases lung edema during high-tidal-volume ventilation, and this effect is completely inhibited by a blocking antibody against the alpha(v)beta6 integrin. Instillation of TFLLRN during high-tidal-volume ventilation is associated with increased pulmonary TGF-beta activation; however, this is not observed in Itgb6-/- mice. Furthermore, Itgb6-/- mice are also protected from ventilator-induced lung edema. We also demonstrate that pulmonary edema and TGF-beta activity are similarly reduced in Par1-/- mice following bleomycin-induced lung injury. These results suggest that PAR1-mediated enhancement of alpha(v)beta6-dependent TGF-beta activation could be one mechanism by which activation of the coagulation cascade contributes to the development of acute lung injury, and they identify PAR1 and the alpha(v)beta6 integrin as potential therapeutic targets in this condition.
Figures
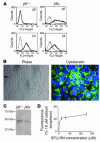
Figure 1. IMLE cells express epithelial cell markers and functional PAR1.
(A) β6 and β4 integrin subunit cell surface expression on Itgb6–/– Immortomouse cells was analyzed before (β_6–/–_) and after (β_6+_) infection with a retroviral vector encoding human WT β6. (B) Epithelial cell morphology was observed by phase-contrast light microscopy (×100), and expression of the epithelial marker cytokeratin was determined by immunofluorescence using a FITC-labeled primary antibody (×600). (C) Presence of the PAR1 receptor in IMLE cells was analyzed by Western blotting of cell lysates using a specific anti-PAR1 antibody. (D) To determine whether the PAR1 in these cells was functional, cell suspensions were treated with increasing doses of the PAR1-activating peptide SFLLRN, and increasing calcium mobilization was determined as a percentage of the positive control, 10 μM calcium ionophore.
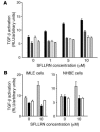
Figure 2. Stimulation of lung epithelial cells with a PAR1 agonist leads to αv β6 -dependent TGF-β activation.
(A) IMLE cells were cocultured with TML cells and stimulated with increasing doses of SFLLRN, and luciferase activity from the TGF-β–responsive plasminogen activator inhibitor-1 promoter was measured. IMLE cells expressing human WT β6 (black bars) were compared with IMLE cells that had no cell surface β6 (white bars), and both were also stimulated with increasing doses of SFLLRN in the presence of αvβ6 blocking antibody (IMLE β6-positive, dark gray bars; IMLE β6-negative, light gray bars). (B) To determine the proportion of TGF-β expression that was mediated by αvβ6 in epithelial cell cultures, IMLE cells and normal human bronchial epithelial (NHBE) cells were stimulated with 10 μM of SFLLRN (gray bars), in the absence or presence of an αvβ6 blocking antibody (black bars) or a pan–TGF-β blocking antibody (white bars), in coculture with TML cells, and luciferase activity was measured. All experiments were performed in triplicate, and the mean + SEM is shown. Results are a representative example of at least 2 identical independent experiments.
Figure 3. PAR1 agonists lead to a time-dependent increase in Smad2 phosphorylation that is αv β6 integrin dependent, but independent of new protein synthesis.
(A and B) The time course of Smad2 phosphorylation in response to 10 μM PAR1-activating peptide SFLLRN (A) and 10 nM thrombin (B), in the presence or absence of an αvβ6 blocking antibody, was assessed by immunoblotting. Total Smad2 levels over time and in response to the αvβ6 blocking antibody were assessed as control. (C) Immunoblot showing the effect of the protein synthesis inhibitor cycloheximide (CHX) on SFLLRN-induced Smad2 phosphorylation. The effectiveness of cycloheximide as a protein synthesis inhibitor was determined by immunoblotting for total Smad2, and immunoblotting for MyD88 was used as a loading control.
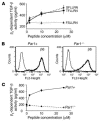
Figure 4. PAR1 peptide–induced αv β6 -dependent TGF-β activity is PAR1 receptor specific.
(A) αvβ6-dependent TGF-β activity following stimulation of mouse embryonic fibroblast cells, stably transfected with human WT β6, with increasing doses of the PAR1-activating peptides TFLLRN and SFLLRN or the scrambled peptide control (FSLLRN), which has no known PAR1-activating effect. αvβ6-dependent TGF-β activity was calculated from coculture bioassays with TML cells, by comparison of the difference in luciferase activity in the absence and presence of αvβ6 blocking antibody with values obtained from a standard curve performed in coculture experiments with increasing concentrations of recombinant TGF-β. (B) After infection with a retroviral vector expressing WT human β6, αvβ6 expression in lung fibroblasts from Par1–/– mice that were either null (Par1–/–) or reconstituted with WT PAR1 (Par1+) was assessed by flow cytometry. (C) αvβ6-expressing Par1–/– and Par1+ fibroblasts were stimulated with increasing doses of SFLLRN, and αvβ6-dependent TGF-β activity was measured by coculture bioassay.
Figure 5. PAR1 signals via RhoA and Rho kinase to induce αv β6 -mediated TGF-β activation.
(A) WT mouse embryonic fibroblasts expressing αvβ6 (MEFβ6) were adenovirally infected with GFP, constitutively active (CA) RhoA, or dominant-negative (DN) RhoA. After infection and cell sorting, cells were stimulated (white bars) or were not stimulated (black bars) with 10 μM SFLLRN, and β6-dependent TGF-β activity was calculated from coculture bioassays with TML cells. Unstimulated Par1–/– cells expressing αvβ6 were also infected with an adenovirus encoding a constitutively active RhoA, dominant-negative RhoA, or GFP control and studied in coculture bioassays, as above. (B) IMLE cells and mouse embryonic fibroblasts, both expressing the β6 integrin, were stimulated with 10 μM SFLLRN (dashed lines) in the presence of increasing doses of the Rho kinase inhibitor Y-27632, and αvβ6-mediated TGF-β activity was compared with that of unstimulated IMLE cells (solid lines). (C) MEFβ6 cells were stimulated with 10 μM SFLLRN or 500 pg/ml TGF-β for 4 hours in the presence or absence of the Rho kinase inhibitor Y-27632 and an αvβ6 blocking antibody, and compared with unstimulated cells. Cell lysates were analyzed by Western blotting for phospho-Smad2 or total Smad2. All results are representative of at least 3 independent experiments. *P < 0.005.
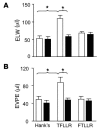
Figure 6. PAR1-induced pulmonary edema is mediated by the αv β6 integrin.
(A) Mice were pretreated with control nonblocking antibody (white bars) or αvβ6 blocking antibody (black bars) before ventilation at 24 ml/kg and intratracheal instillation with PAR1-specific peptide (TFLLRN), vehicle (HBSS), or peptide control (FTLLRN). ELW was measured as the wet-to-dry weight ratio of excised lungs. (B) Mice were pretreated with control nonblocking antibody (white bars) or αvβ6 blocking antibody (black bars) before ventilation at 24 ml/kg and intratracheal instillation of peptide and controls. Lung extravascular plasma equivalents were calculated based on the extravasation of 125I-labeled albumin into the lungs. Hank’s, n = 8; TFLLRN, n = 10; and FTLLRN, n = 11. Values are presented as mean + SEM. *P < 0.01.
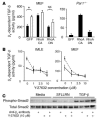
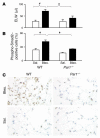
Figure 7. Itgb6–/– mice instilled with TFLLRN and ventilated at high tidal volume are protected from lung edema and have reduced TGF-β activity in the lung compared with WT mice.
(A) WT mice (white bars) and Itgb6–/– mice (black bars) were ventilated and instilled with peptide and control, and lung edema was measured as described previously. WT, n = 6; and Itgb6–/–, n = 5. (B) Quantification of nuclear localized phospho-Smad2 immunostaining in WT mice instilled with Hank’s or PAR1-activating peptide or Itgb6–/– mice instilled with PAR1-activating peptide (black bar). WT mice, Hank’s, n = 3; WT mice, TFLLRN, n = 4; Itgb6–/– mice, n = 3; and Par1–/– mice, saline, n = 3. (C) Representative histological sections showing nuclear localized phospho-Smad2 immunostaining in the lung of WT or Itgb6–/– mice. Original magnification, ×400. Values are presented as mean + SEM. *P < 0.05.
Figure 8. Par1–/– mice instilled with intratracheal bleomycin have reduced permeability and TGF-β activation compared with WT control.
(A) WT or Par1–/– mice were instilled with saline (Sal.; white bars) or 0.05 U bleomycin (Bleo.; black bars). ELW was measured as described. Bleomycin, n = 6; and saline, n = 5. (B) Quantification of nuclear localized phospho-Smad2 immunostaining in WT or Par1–/– mice instilled with saline (white bars) or 0.05 U bleomycin (black bars). WT mice, n = 4; Par1–/–, bleomycin, n = 4; and Par1–/–, saline, n = 3. (C) Representative histological sections showing nuclear localized phospho-Smad2 immunostaining in the lung of WT or Par1–/– mice. Original magnification, ×400. Values are presented as mean + SEM. #P < 0.005, *P < 0.05.
Similar articles
- Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms.
Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, Matthay MA, Pittet JF. Ganter MT, et al. Circ Res. 2008 Apr 11;102(7):804-12. doi: 10.1161/CIRCRESAHA.107.161067. Epub 2008 Feb 14. Circ Res. 2008. PMID: 18276918 Free PMC article. - Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis.
Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Puthawala K, et al. Am J Respir Crit Care Med. 2008 Jan 1;177(1):82-90. doi: 10.1164/rccm.200706-806OC. Epub 2007 Oct 4. Am J Respir Crit Care Med. 2008. PMID: 17916808 Free PMC article. - Role of integrin alphav beta6 in acute lung injury induced by Pseudomonas aeruginosa.
Song Y, Pittet JF, Huang X, He H, Lynch SV, Violette SM, Weinreb PH, Horan GS, Carmago A, Sawa Y, Bernstein XL, Wiener-Kronish JP. Song Y, et al. Infect Immun. 2008 Jun;76(6):2325-32. doi: 10.1128/IAI.01431-07. Epub 2008 Mar 31. Infect Immun. 2008. PMID: 18378634 Free PMC article. - Signaling Crosstalk of TGF-β/ALK5 and PAR2/PAR1: A Complex Regulatory Network Controlling Fibrosis and Cancer.
Ungefroren H, Gieseler F, Kaufmann R, Settmacher U, Lehnert H, Rauch BH. Ungefroren H, et al. Int J Mol Sci. 2018 May 24;19(6):1568. doi: 10.3390/ijms19061568. Int J Mol Sci. 2018. PMID: 29795022 Free PMC article. Review. - Epithelial-mesenchymal interactions in fibrosis and repair. Transforming growth factor-β activation by epithelial cells and fibroblasts.
Sheppard D. Sheppard D. Ann Am Thorac Soc. 2015 Mar;12 Suppl 1(Suppl 1):S21-3. doi: 10.1513/AnnalsATS.201406-245MG. Ann Am Thorac Soc. 2015. PMID: 25830829 Free PMC article. Review.
Cited by
- αv integrins: key regulators of tissue fibrosis.
Conroy KP, Kitto LJ, Henderson NC. Conroy KP, et al. Cell Tissue Res. 2016 Sep;365(3):511-9. doi: 10.1007/s00441-016-2407-9. Epub 2016 May 2. Cell Tissue Res. 2016. PMID: 27139180 Free PMC article. Review. - Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells.
Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ. Burch ML, et al. J Biol Chem. 2013 Mar 8;288(10):7410-9. doi: 10.1074/jbc.M112.400259. Epub 2013 Jan 18. J Biol Chem. 2013. PMID: 23335513 Free PMC article. - Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin α(v)β(6)-dependent physical force.
Giacomini MM, Travis MA, Kudo M, Sheppard D. Giacomini MM, et al. Exp Cell Res. 2012 Apr 1;318(6):716-22. doi: 10.1016/j.yexcr.2012.01.020. Epub 2012 Jan 28. Exp Cell Res. 2012. PMID: 22309779 Free PMC article. - Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury.
Yasui H, Donahue DL, Walsh M, Castellino FJ, Ploplis VA. Yasui H, et al. Am J Physiol Lung Cell Mol Physiol. 2016 Jul 1;311(1):L74-86. doi: 10.1152/ajplung.00429.2015. Epub 2016 May 17. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27190065 Free PMC article. - Protease-activated receptor-1 impairs host defense in murine pneumococcal pneumonia: a controlled laboratory study.
Schouten M, van't Veer C, Roelofs JJ, Levi M, van der Poll T. Schouten M, et al. Crit Care. 2012 Dec 27;16(6):R238. doi: 10.1186/cc11910. Crit Care. 2012. PMID: 23270594 Free PMC article.
References
- Munger J.S., et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. - PubMed
- Lawrence D.A., Pircher R., Jullien P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem. Biophys. Res. Commun. 1985;133:1026–1034. - PubMed
- Pircher R., Jullien P., Lawrence D.A. Beta-transforming growth factor is stored in human blood platelets as a latent high molecular weight complex. Biochem. Biophys. Res. Commun. 1986;136:30–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL51856/HL/NHLBI NIH HHS/United States
- HL53949/HL/NHLBI NIH HHS/United States
- R01 HL064353/HL/NHLBI NIH HHS/United States
- R01 HL051856/HL/NHLBI NIH HHS/United States
- R01 HL053949/HL/NHLBI NIH HHS/United States
- R37 HL051856/HL/NHLBI NIH HHS/United States
- R01 HL051854/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- HL51854/HL/NHLBI NIH HHS/United States
- P50 HL056385/HL/NHLBI NIH HHS/United States
- HL74005/HL/NHLBI NIH HHS/United States
- R37 HL053949/HL/NHLBI NIH HHS/United States
- P50 HL074005/HL/NHLBI NIH HHS/United States
- HL56385/HL/NHLBI NIH HHS/United States
- HL66600/HL/NHLBI NIH HHS/United States
- U01 HL066600/HL/NHLBI NIH HHS/United States
- HL64353/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases