Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis - PubMed (original) (raw)
Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis
Fang Yang et al. J Cell Biol. 2006.
Abstract
During meiosis, the arrangement of homologous chromosomes is tightly regulated by the synaptonemal complex (SC). Each SC consists of two axial/lateral elements (AEs/LEs), and numerous transverse filaments. SC protein 2 (SYCP2) and SYCP3 are integral components of AEs/LEs in mammals. We find that SYCP2 forms heterodimers with SYCP3 both in vitro and in vivo. An evolutionarily conserved coiled coil domain in SYCP2 is required for binding to SYCP3. We generated a mutant Sycp2 allele in mice that lacks the coiled coil domain. The fertility of homozygous Sycp2 mutant mice is sexually dimorphic; males are sterile because of a block in meiosis, whereas females are subfertile with sharply reduced litter size. Sycp2 mutant spermatocytes exhibit failure in the formation of AEs and chromosomal synapsis. Strikingly, the mutant SYCP2 protein localizes to axial chromosomal cores in both spermatocytes and fetal oocytes, but SYCP3 does not, demonstrating that SYCP2 is a primary determinant of AEs/LEs and, thus, is required for the incorporation of SYCP3 into SCs.
Figures
Figure 1.
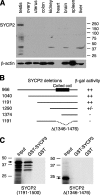
Characterization of SYCP2 expression and its interaction with SYCP3. (A) Western blot analysis of SYCP2 in adult mouse tissues. 30 μg of protein extracts were loaded. β-actin served as a loading control. Rabbit anti-SYCP2 polyclonal antibodies were used (1:500). (B) Identification of SYCP3-binding domain. Various truncated SYCP2 polypeptides were cloned in the pACT2 vector and tested for interaction with full-length mouse SYCP3 in the pAS2-1 vector by yeast two-hybrid β-galactosidase filter assay. ++, dark blue within 3 h of incubation; +/−, light blue within 5 h; −, not blue after overnight incubation. Numbers indicate the position of terminal residues in each clone. The coiled coil region is shown as a black box. (C) In vitro GST pulldown assay. Full-length SYCP3 protein (254 aa) was expressed as a GST fusion protein and affinity purified. The SYCP2 polypeptides (residues 1,191–1,500) with or without the deletion (residues 1,346–1,476) were translated in vitro and tested for binding with GST-SYCP3. Arrows indicate the in vitro–translated SYCP2 polypeptides. Molecular mass standards are shown in kilodaltons.
Figure 2.
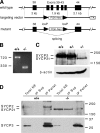
Targeted disruption of the Sycp2 gene. (A) Schematic diagram of the Sycp2 targeting strategy. Neomycin selection marker (PGK-Neo) was flanked by loxP sites. Thymidine kinase (TK) was included for negative selection with ganciclovir. Exons are shown in black boxes and designated by the numbers shown above the boxes. (B) Splicing of the mutant Sycp2 transcript. RT-PCR was done with poly(A)+ RNAs from wild-type (+/+) and Sycp2 mutant (−/−) testes. PCR primers are located in exons 38 and 44. PCR product size is shown in bps. (C) Production of the mutant SYCP2t protein. Western blot analysis was performed on wild-type, Sycp2 +/−, and Sycp2 −/− testicular protein extracts using rabbit anti-SYCP2 serum. (D) In vivo association of SYCP3 with SYCP2, but not SYCP2t. Soluble nuclear protein extracts from wild-type and Sycp2 −/− testes were immunoprecipitated with anti-SYCP3 antibodies. Immunoblotting was probed with anti-SYCP2 antibodies. SYCP2, but not SYCP2t, was coimmunoprecipitated with SYCP3. *, a nonspecific diffuse band observed in the immunoprecipitated (IP) pellet of both wild-type and mutant. IP, immunoprecipitation; NE, nuclear extract; Sup, supernatant. Protein molecular mass standards are shown in kilodaltons.
Figure 3.
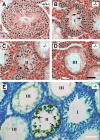
Meiotic arrest and apoptosis of spermatocytes in _Sycp2_−/− mice. Testes from 8-wk-old wild-type and Sycp2 −/− mice were used for histological and apoptosis analyses. (A) Wild-type seminiferous tubule contains a full spectrum of germ cells: spermatogonia, pachytene spermatocytes (PS), round spermatids (RS), and elongated spermatids (ES). (B–D) Three types of tubules observed in Sycp2 −/− testis. (B) In type I tubules, multiple layers of zygotene-like spermatocytes (arrows) are present. (C) In type II tubules, multiple layers of heavily eosin-stained cells (arrows) are present. (D) In type III tubules, only a single layer of spermatogonia and Sertoli cells are present. (E) Apoptosis of spermatocytes in type II Sycp2 −/− tubules. Arrows indicate one or two layers of brown-stained apoptotic cells. Bars, 50 μm.
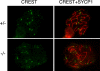
Figure 4.
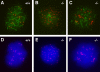
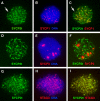
Failure of chromosome synapsis in _Sycp2_−/− spermatocytes. Spermatocytes from wild-type (A and D) and knockout (B, C, E, and F) mice were stained with anti-SYCP1 serum (green), CREST antiserum (red), and DAPI (blue). (A and D) In wild-type pachytene spermatocytes, SYCP1 labels SCs that have formed along synapsed homologous chromosomes. CREST antiserum marks 21 foci. (B and E) Early Sycp2 −/− spermatocytes contain 40 CREST foci, suggesting that the cohesion of sister chromatids is not affected, at least not in the centromeric regions. SYCP1 is expressed, but apparently not in the form of fibers. (C and F) Many short SYCP1 fibers are present in advanced Sycp2 −/− spermatocytes. There are ∼34 CREST foci in this nucleus. Note that the CREST foci often exist in pairs.
Figure 5.
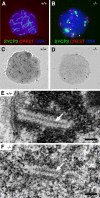
Analysis of SC formation in _Sycp2_−/− spermatocytes. Surface-spread nuclei were stained with anti-SYCP3 antibodies, CREST, and DAPI, or they were stained with silver nitrate. (A) SYCP3 labels SCs in wild-type spermatocytes. (B) SYCP3 fails to incorporate into SCs in Sycp2 −/− spermatocytes. SYCP3 forms several large protein aggregates (arrows). In addition, SYCP3 also forms many small nuclear foci (arrowhead). (C) SCs in wild-type spermatocytes are readily stained with silver nitrate. (D) No silver-stained SC structures are visible by light microscopy in Sycp2 −/− spermatocytes. (E) EM analysis of a wild-type spermatocyte. A tripartite structure of SC is formed by one CE (arrow) that connects with two electron-dense LEs. This SC is attached to the nuclear envelope (NE). Note the condensed chromatin around the SC. (F) EM analysis of a Sycp2 −/− spermatocyte. TFs form a CE-like structure (arrow). Two rows of chromatin grains align in parallel with the CE-like structure to form a SC-like structure. Bars, 0.2 μm.
Figure 6.
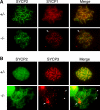
Association of SYCP2t with axial chromosomal cores in _Sycp2_−/− spermatocytes. Surface-spread nuclei of Sycp2 −/− spermatocytes were immunostained with guinea pig anti-SYCP2 serum and anti-SYCP1, or anti-SYCP3, or anti-STAG3 antibodies. DNA was stained with DAPI. Arrows (A and C) indicate a fine fiber with a bead-on-a-string appearance. The three images in each row represent the same Sycp2 −/− spermatocyte.
Figure 7.
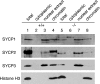
Association of SC proteins with chromatin. Cytoplasmic, nuclear, and chromatin extracts were sequentially prepared from wild-type and Sycp2 −/− testes. Equal fractions of nuclear and chromatin extracts were loaded. The same preparations were subjected to Western blot analysis with antibodies against SYCP1, -2, -3, and histone H3. Histone H3 is a core histone and, thus, is tightly bound to chromatin.
Figure 8.
Localization of SYCP1, 2 and 3 in _Sycp2_−/− oocytes. Oocytes from embryonic ovaries at 17.5 dpc were analyzed by immunostaining. Sycp2 +/− littermates were used as controls. (A) SYCP1 and -2 localize to fully synapsed and continuous SCs in a Sycp2 +/− pachytene oocyte. However, axial gaps and interrupted synapsis are prominent in Sycp2 −/− oocytes, as indicated by arrows (this oocyte is at the late pachytene/early diplotene stage). (B) Localization of SYCP3 in Sycp2 −/− oocytes. SYCP3 fails to localize to AEs, but instead forms a few large nuclear aggregates (arrow). In addition, many small SYCP3 foci are present at chromosome ends (arrowheads).
Figure 9.
Pairing of homologous chromosomes in _Sycp2_−/− oocytes. Sycp2 +/− and Sycp2 −/− oocytes at 17.5 dpc were stained with CREST antiserum (green) and anti-SYCP1 antibodies (red). Control pachytene stage oocyte exhibited 20 CREST foci (centromeres) and full synapsis of homologous chromosomes (top). Chromosome pairing was present in Sycp2 −/− oocytes (bottom).
Similar articles
- Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis.
Fukuda T, Pratto F, Schimenti JC, Turner JM, Camerini-Otero RD, Höög C. Fukuda T, et al. PLoS Genet. 2012 Feb;8(2):e1002485. doi: 10.1371/journal.pgen.1002485. Epub 2012 Feb 9. PLoS Genet. 2012. PMID: 22346761 Free PMC article. - Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis.
Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, Wang PJ, Meistrich ML, Rajkovic A. Shin YH, et al. PLoS Genet. 2010 Nov 4;6(11):e1001190. doi: 10.1371/journal.pgen.1001190. PLoS Genet. 2010. PMID: 21079677 Free PMC article. - Meiotic interference among MLH1 foci requires neither an intact axial element structure nor full synapsis.
de Boer E, Dietrich AJ, Höög C, Stam P, Heyting C. de Boer E, et al. J Cell Sci. 2007 Mar 1;120(Pt 5):731-6. doi: 10.1242/jcs.003186. Epub 2007 Feb 13. J Cell Sci. 2007. PMID: 17298983 - Mutations in Genes Coding for Synaptonemal Complex Proteins and Their Impact on Human Fertility.
Geisinger A, Benavente R. Geisinger A, et al. Cytogenet Genome Res. 2016;150(2):77-85. doi: 10.1159/000453344. Epub 2016 Dec 21. Cytogenet Genome Res. 2016. PMID: 27997882 Review. - Meiotic Chromosome Structure, the Synaptonemal Complex, and Infertility.
Adams IR, Davies OR. Adams IR, et al. Annu Rev Genomics Hum Genet. 2023 Aug 25;24:35-61. doi: 10.1146/annurev-genom-110122-090239. Epub 2023 May 9. Annu Rev Genomics Hum Genet. 2023. PMID: 37159901 Review.
Cited by
- Defects in meiosis I contribute to the genesis of androgenetic hydatidiform moles.
Rezaei M, Liang M, Yalcin Z, Martin JH, Kazemi P, Bareke E, Ge ZJ, Fardaei M, Benadiva C, Hemida R, Hassan A, Maher GJ, Abdalla E, Buckett W, Bolze PA, Sandhu I, Duman O, Agrawal S, Qian J, Vallian Broojeni J, Bhati L, Miron P, Allias F, Selim A, Fisher RA, Seckl MJ, Sauthier P, Touitou I, Tan SL, Majewski J, Taketo T, Slim R. Rezaei M, et al. J Clin Invest. 2024 Nov 15;134(22):e170669. doi: 10.1172/JCI170669. J Clin Invest. 2024. PMID: 39545410 Free PMC article. - The Abl1 tyrosine kinase is a key player in doxorubicin-induced cardiomyopathy and its p53/p73 cell death mediated signaling differs in atrial and ventricular cardiomyocytes.
Borlak J, Ciribilli Y, Bisio A, Selvaraj S, Inga A, Oh JH, Spanel R. Borlak J, et al. J Transl Med. 2024 Sep 16;22(1):845. doi: 10.1186/s12967-024-05623-8. J Transl Med. 2024. PMID: 39285385 Free PMC article. - TRIP13 localizes to synapsed chromosomes and functions as a dosage-sensitive regulator of meiosis.
Chotiner JY, Leu NA, Yang F, Cossu IG, Guan Y, Lin H, Wang PJ. Chotiner JY, et al. Elife. 2024 Aug 29;12:RP92195. doi: 10.7554/eLife.92195. Elife. 2024. PMID: 39207914 Free PMC article. - Novel Loss-of-Function SYCP2 Variants in Infertile Males Upgrade the Gene-Disease Clinical Validity Classification for SYCP2 and Male Infertility to Strong.
Li J, Schilit SLP, Liang S, Qin N, Teng X, Zhang J. Li J, et al. Genes (Basel). 2024 Aug 19;15(8):1092. doi: 10.3390/genes15081092. Genes (Basel). 2024. PMID: 39202451 Free PMC article. - The N-terminal modification of HORMAD2 causes its ectopic persistence on synapsed chromosomes without meiotic blockade.
Cossu IG, Leu NA, Guan Y, Wang PJ. Cossu IG, et al. Reproduction. 2024 Mar 13;167(4):e230330. doi: 10.1530/REP-23-0330. Print 2024 Apr 1. Reproduction. 2024. PMID: 38401263
References
- Chuma, S., and N. Nakatsuji. 2001. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Dev. Biol. 229:468–479. - PubMed
- Colaiacovo, M.P., A.J. MacQueen, E. Martinez-Perez, K. McDonald, A. Adamo, A. La Volpe, and A.M. Villeneuve. 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell. 5:463–474. - PubMed
- Costa, Y., R. Speed, R. Ollinger, M. Alsheimer, C.A. Semple, P. Gautier, K. Maratou, I. Novak, C. Hoog, R. Benavente, and H.J. Cooke. 2005. Two novel proteins recruited by synaptonemal complex protein 1 (SYCP1) are at the centre of meiosis. J. Cell Sci. 118:2755–2762. - PubMed
- de los Santos, T., and N.M. Hollingsworth. 1999. Red1p, a MEK1-dependent phosphoprotein that physically interacts with Hop1p during meiosis in yeast. J. Biol. Chem. 274:1783–1790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD042740/HD/NICHD NIH HHS/United States
- U01 HD045866/HD/NICHD NIH HHS/United States
- U01 HD045866-03/HD/NICHD NIH HHS/United States
- HD042740/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases