Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics - PubMed (original) (raw)
Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics
Anjana Nayal et al. J Cell Biol. 2006.
Abstract
Continuous adhesion formation and disassembly (adhesion turnover) in the protrusions of migrating cells is regulated by unclear mechanisms. We show that p21-activated kinase (PAK)-induced phosphorylation of serine 273 in paxillin is a critical regulator of this turnover. Paxillin-S273 phosphorylation dramatically increases migration, protrusion, and adhesion turnover by increasing paxillin-GIT1 binding and promoting the localization of a GIT1-PIX-PAK signaling module near the leading edge. Mutants that interfere with the formation of this ternary module abrogate the effects of paxillin-S273 phosphorylation. PAK-dependent paxillin-S273 phosphorylation functions in a positive-feedback loop, as active PAK, active Rac, and myosin II activity are all downstream effectors of this turnover pathway. Finally, our studies led us to identify in highly motile cells a class of small adhesions that reside near the leading edge, turnover in 20-30 s, and resemble those seen with paxillin-S273 phosphorylation. These adhesions appear to be regulated by the GIT1-PIX-PAK module near the leading edge.
Figures
Figure 1.
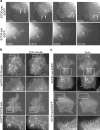
S273-paxillin phosphorylation by PAK regulates paxillin–GIT1 binding. (a) CHO-K1 lysates treated (right) and untreated (left) with 5 nM CalyculinA (CalA) were probed (top) using a phospho–S273-paxillin–specific antibody. Total paxillin levels were assayed with an anti-paxillin antibody (bottom). A single band corresponding to the molecular mass of paxillin (∼68 kD) was detected in treated lysates. (b) Kinase assay was performed with FLAG-WT-paxillin and either KD- or CA-myc-PAK synthesized in vitro, and S273-paxillin phosphorylation was assessed with a phospho–S273-paxillin antibody. Bottom blots show equal loading by probing with anti-FLAG and anti-myc antibodies, respectively. Phospho–S273-paxillin levels increased eightfold with CA-PAK compared with KD-PAK. (c) Paxillin was immunoprecipitated using a GFP antibody from CHO-K1 lysates expressing paxillin-GFP and either KD- or CA-myc-PAK, and S273-paxillin phosphorylation levels were assayed using a phospho–S273-paxillin antibody. The lower two panels show equal levels of paxillin–GFP and myc-PAK, and the GFP blot shows equal loading in the lysates. S273-paxillin phosphorylation increased eightfold with CA-PAK as compared with KD-PAK. (d) A GFP antibody was used to immunoprecipitate paxillin from CHO-K1 lysates expressing GFP control or WT-, S273A-, or S273D-paxillin-GFP and FLAG-GIT1. GIT1 binding was probed using an anti-FLAG antibody. The bottom two panels show equivalent expression of S273-paxillin mutants and FLAG-GIT1 in the lysates. GIT1 binding to S273D-paxillin increased threefold, whereas it was reduced twofold with S273A-paxillin, when compared with WT-paxillin. (e) Paxillin was immunoprecipitated from CHO-K1 lysates expressing GFP control or WT-, S273A-, or S273D-paxillin-GFP and myc-FAK using a GFP antibody, and FAK binding was assessed with an anti-myc antibody. The bottom two panels show equivalent expression of S273-paxillin mutants and myc-FAK in the lysates. S273-paxillin phosphorylation only marginally affected FAK binding. (f) GIT1 was immunoprecipitated from in vitro mixtures of FLAG-GIT1, untagged WT-paxillin, and either KD- or CA-PAK using anti-FLAG M2-conjugated agarose, and phospho–S273-paxillin binding was probed using a phospho–S273-paxillin antibody. The middle blot shows equal levels of FLAG-GIT1 using an anti-FLAG antibody. (bottom) Equal loading of the lysates using anti-myc and anti-paxillin antibodies, respectively. Phospho–S273-paxillin–GIT1 binding increased sevenfold with CA-PAK compared with KD-PAK. (g) Anti-FLAG M2-conjugated agarose was used to immunoprecipitate GIT1 from in vitro mixtures of FLAG-GIT1, untagged WT-paxillin, and CA-PAK preincubated with 500-fold molar excess of phospho– or nonphospho–S273-paxillin peptide, and phospho–S273-paxillin binding was assessed with a phospho–S273-paxillin antibody. Very low levels of phospho–S273-paxillin–GIT1 binding was detected with the competitive phosphopeptide (left), whereas a robust signal was observed with the noncompetitive peptide (right), confirming that the PAK-mediated increase in phospho–S273-paxillin–GIT1 binding is specific to S273-paxillin phosphorylation.
Figure 2.
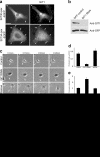
S273-paxillin phosphorylation increases cell migration and protrusiveness. (a) Wind rose plots for CHO-K1 cells expressing WT-, S273A- or S273D-paxillin-GFP. S273A-paxillin expression led to shorter migration paths (middle), whereas those for S273D-paxillin were significantly longer (right) compared with WT-paxillin (left). The plots show data from nine representative cells from three independent experiments. (b) Kymographs from CHO-K1 cells expressing WT-, S273A-, or S273D-paxillin-GFP. Cell edges were enhanced using the Sobel algorithm in the Fluoview software. Cells expressing S273D-paxillin-GFP show rapid membrane extension and retraction (bottom) when compared with cells expressing S273A-paxillin-GFP (middle). WT-paxillin-GFP–expressing cells show intermediate membrane activity (top). Bars: (vertical) 5 μm; (horizontal) 5 min. (c) Quantification of protrusion rates from kymographs. S273D-paxillin mutant increases, whereas S273A-paxillin reduces the protrusion rate with respect to WT-paxillin. (d) Protrusion stability in S273D-paxillin–expressing cells decreased, whereas it increased in S273A-paxillin–expressing cells. A minimum of eight cells and at least three protrusions per cell from three independent experiments were analyzed for each kymograph analysis. Error bars represent SEM from three experiments.
Figure 3.
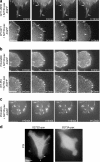
S273-paxillin phosphorylation induces the formation of small, dynamic adhesions in protrusions. (a, top) Time-lapse imaging of CHO-K1 cells showed S273D-paxillin-GFP localization in small adhesions that appeared and disappeared (turned over) in 1–2 frames (arrows) near the leading edge. (bottom) On the other hand, S273A-paxillin-GFP localized into large adhesions (arrow) near the base of the protrusion that either tend to slide or disassemble slowly (Table I). (b, top) seCFP-S273D-paxillin and YFP-vinculin exhibit colocalization in small adhesions within protrusions (arrows) of CHO-K1 cells, imaged by TIRF. Enlargements of the boxed regions are at the bottom of each panel. Rates of paxillin and vinculin disassembly in these adhesions were accelerated similarly (Table I), indicating that fast turnover is a property of the entire adhesion. (bottom) In contrast, CHO-K1 cells coexpressing seCFP-S273A-paxillin and YFP-vinculin exhibited larger adhesions (arrows) with similar but slower adhesion disassembly rates (Table I). Bar, 20 μm. (c) S273A- and S273D-paxillin-GFP colocalized with zyxin in small adhesions in protrusive parts of CHO-K1 cells and in larger adhesions at the protrusion base, respectively, as shown in TIRF experiments. Arrows point to the regions where paxillin and zyxin colocalize.
Figure 4.
GIT1 is targeted near the leading edge on S273-paxillin phosphorylation, where it enhances protrusion and adhesion dynamics. (a, left) Subcellular localization of the paxillin-GFP mutants is shown. (right) GIT1 immunostaining showed that both paxillin and GIT1 localized prominently near the leading edge (arrows) in CHO-K1 cells expressing S273D-paxillin-GFP. However, in S273A-paxillin-GFP–expressing CHO-K1 cells, GIT1 localized weakly in only some of the large adhesions (arrows) but not in others (arrowheads). Bar, 10 μm. (b) Immunoblot of Rat2 lysates coexpressing GFP and either pSUPER vector (control) or GIT1 RNAi. The blot was probed with a GIT1 antibody. GIT1 RNAi, but not pSUPER alone, caused a large decrease in endogenous GIT1 expression. (c) GIT1 knockdown decreases protrusiveness compared with the pSUPER control (arrows show stable and dynamic protrusions, respectively). Coexpression of human GIT1 with rat GIT1 RNAi (rescue) restored the protrusiveness (arrow). Bar, 30 μm. (d) Quantification of protrusion rate. GIT1 RNAi decreased the protrusion rate compared with the control, and this defect was rescued by coexpressing GFP-tagged human GIT1 with rat GIT1 RNAi (rescue). (e) GIT1 knockdown increased protrusion stability compared with control cells, whereas coexpression of GFP-tagged human GIT1 (rescue) decreased it back to control levels. A minimum of eight cells per treatment and at least three protrusions per cell from three independent experiments were analyzed. Error bars represent SEM from three experiments. (f) GIT1 knockdown reduces adhesion turnover. Note that the large adhesions near the cell periphery (arrows) in Rat2 cells coexpressing GIT1 RNAi are more stable than WT-paxillin-GFP–expressing cells. Control cells expressing WT-paxillin-GFP and the pSUPER vector alone show smaller and more dynamic adhesions near the cell periphery (arrows). Bar, 5 μm. (g) Phospho–S273-paxillin immunostaining showed robust localization of phospho–S273-paxillin near the leading edge in S273D-paxillin-GFP–expressing cells but was not readily detected in S273A-paxillin-GFP–expressing cells. Bar, 10 μm. (h) CHO-K1 cells were immunostained for endogenous phospho–S273-paxillin and visualized using TIRF. Endogenous phospho–S273-paxillin localized in small puncta near the leading edge. Bar, 10 μm.
Figure 5.
PAK activation and localization to the leading edge is required for fast adhesion dynamics. (a) KD-PAK strongly inhibited the S273D-paxillin phenotype. CHO-K1 cells coexpressing KD-PAK and S273D-paxillin-GFP formed large adhesions (arrows) with a reduced disassembly rate. Bar, 5 μm. (b) PAK activation enhances adhesion turnover. (top) CHO-K1 cells coexpressing KD-PAK and WT-paxillin-GFP show large and stable adhesions (arrows) that disassemble slowly (Table II). (bottom) In contrast, note the numerous small and dynamic adhesions (arrows) near the leading edge in cells coexpressing CA-PAK and WT-paxillin-GFP. These adhesions formed and disassembled very rapidly (Table II). Bar, 5 μm. (c) Phospho-PAK immunostaining in CHO-K1 cells expressing S273D- or S273A-paxillin-GFP. The S273D mutant showed robust phospho-PAK localization near the leading edge (arrows), whereas the labeling pattern was diffuse in cells expressing S273A-paxillin. Bar, 20 μm. (d) CHO-K1 cells coexpressing CA-PAK and WT-paxillin-GFP were treated with blebbistatin, a myosin ATPase inhibitor. (top) Small adhesions (arrows) turn over rapidly in the protrusive regions of the cell before exposure to blebbistatin. (middle) Immediately after exposure, protrusion ceased and the adhesions stopped turning over (arrows). (bottom) After washout of blebbistatin, faster adhesion turnover recovered (arrows). Bar, 5 μm.
Figure 6.
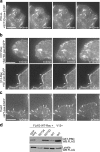
PIX–GIT1 and PIX–PAK interactions are required for increased protrusion and adhesion dynamics. (a, top) GIT1ΔSHD coexpression with S273D-paxillin led to the formation of large stable adhesions (arrows; Table III). (bottom) Control CHO-K1 cells coexpressing WT-GIT1 and S273D-paxillin showed small, dynamic adhesions in the protrusive regions of the cell (arrows; Table III). Bar, 5 μm. (b) CHO-K1 cells coexpressing PIXΔGBD and S273D-paxillin show small adhesions that exhibit rapid disassembly (arrows; Table III) in the protrusive regions of these cells. (bottom) Control CHO-K1 cells coexpressing WT-PIX and S273D-paxillin showed small adhesions (arrows) that disassemble very rapidly (Table III) in the protrusive regions of the cell. Bar, 10 μm. (c) Coexpression of PIXΔSH3 with S273D-paxillin in CHO-K1 cells led to the formation of large stable adhesions (arrows; Table III). Bar, 5 μm. (d) Immunostaining for PIX in CHO-K1 cells expressing S273D- or S273A-paxillin-GFP showed robust PIX localization to a region near the leading edge (arrows) in cells expressing S273D-paxillin. In contrast, the leading edge PIX localization was not observed in S273A-paxillin–expressing cells. Bar, 20 μm.
Figure 7.
Rac activation regulates adhesion turnover. (a) CHO-K1 cells coexpressing S273D-paxillin-GFP and a PIX mutant, which lacks nucleotide exchange activity (PIX-LL) showed large adhesions (arrows) that disassembled slowly (Table III). Bar, 5 μm. (b, top) CHO-K1 cells coexpressing dominant-negative (N17-Rac) and S273D-paxillin-GFP show large and stable adhesions (arrows) that disassemble slowly (Table IV). (bottom) CHO-K1 cells coexpressing CA- (V12-Rac) and WT-paxillin-GFP exhibit numerous small but stable adhesions (Table IV) at the cell periphery (arrows). Bar, 5 μm. (c) CHO-K1 cells coexpressing the Rac GEF Tiam1 and WT-paxillin-GFP show numerous small paxillin-containing adhesions (arrows) that disassemble very rapidly (Table IV). Bar, 5 μm. (d) S273-paxillin phosphorylation increases Rac activation. Rac activity from CHO-K1 lysates coexpressing FLAG-WT-Rac and paxillin-GFP mutants was measured using a GST-PBD pull-down assay. CHO-K1 lysates coexpressing FLAG-V12-Rac and WT-paxillin-GFP served as a positive control. (top) Active Rac in GST-PBD bead pellets was detected by immunoblotting using an anti-FLAG antibody. (bottom) Equal protein aliquots of lysates served as loading controls. Rac activation increased eightfold with S273D-paxillin when compared with S273A-paxillin and 1.5-fold compared with WT-paxillin.
Figure 8.
Characterization of the small and dynamic adhesions near the leading edge. (a) TIRF visualization of S273D- and WT-paxillin adhesions. Numerous small adhesions are seen along the leading edge in the S273D- as well as WT-paxillin–expressing cells. Enlargements of the boxed regions are shown in the right panels as inverse images (arrows show small adhesions near the leading edges). Bar, 5 μm. (b) Adhesion turnover assay using high time resolution imaging (time intervals ∼3–5 s). Both S273D- and WT-paxillin containing small adhesions turned over at similar rates. The error bars represent SEM from independent experiments. For each condition, 40–60 adhesions from 8–10 different cells were analyzed. (c, top) CHO-K1 cells coexpressing WT-paxillin-GFP and actin–monomeric RFP showed small adhesions (arrows) near the leading edge ∼1 μm behind the peak intensity of the actin band (compare black circles to open triangles). Bar, 5 μm. (bottom) Coincidence between paxillin, IRM, and DIC images. The leading edge is ahead of the paxillin-containing adhesions and does not overlap with the IRM signal, showing that it is not attached to the substratum. Bar, 2 μm. (d) TIRF visualization of endogenous paxillin and GIT1 in CHO-K1 cells. Both localized in the small adhesions near the leading edge (arrows). Enlargements of the boxed regions are shown in the right panels as inverse images. Bar, 5 μm.
Similar articles
- Phosphorylation of serine 709 in GIT1 regulates protrusive activity in cells.
Webb DJ, Kovalenko M, Whitmore L, Horwitz AF. Webb DJ, et al. Biochem Biophys Res Commun. 2006 Aug 11;346(4):1284-8. doi: 10.1016/j.bbrc.2006.06.036. Epub 2006 Jun 14. Biochem Biophys Res Commun. 2006. PMID: 16797488 - p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase.
Lee J, Jung ID, Chang WK, Park CG, Cho DY, Shin EY, Seo DW, Kim YK, Lee HW, Han JW, Lee HY. Lee J, et al. Exp Cell Res. 2005 Jul 15;307(2):315-28. doi: 10.1016/j.yexcr.2005.03.028. Exp Cell Res. 2005. PMID: 15893751 - Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway.
Brown MC, West KA, Turner CE. Brown MC, et al. Mol Biol Cell. 2002 May;13(5):1550-65. doi: 10.1091/mbc.02-02-0015. Mol Biol Cell. 2002. PMID: 12006652 Free PMC article. - Illuminating adhesion complexes in migrating cells: moving toward a bright future.
Webb DJ, Brown CM, Horwitz AF. Webb DJ, et al. Curr Opin Cell Biol. 2003 Oct;15(5):614-20. doi: 10.1016/s0955-0674(03)00105-4. Curr Opin Cell Biol. 2003. PMID: 14519397 Review. - Pak to the future.
Bagrodia S, Cerione RA. Bagrodia S, et al. Trends Cell Biol. 1999 Sep;9(9):350-5. doi: 10.1016/s0962-8924(99)01618-9. Trends Cell Biol. 1999. PMID: 10461188 Review.
Cited by
- Roles of paxillin phosphorylation in IL-3 withdrawal-induced Ba/F3 cell apoptosis.
Nah AS, Chay KO. Nah AS, et al. Genes Genomics. 2019 Feb;41(2):241-248. doi: 10.1007/s13258-018-00779-2. Epub 2019 Jan 2. Genes Genomics. 2019. PMID: 30604146 - The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration.
Lawson CD, Burridge K. Lawson CD, et al. Small GTPases. 2014;5:e27958. doi: 10.4161/sgtp.27958. Epub 2014 Mar 7. Small GTPases. 2014. PMID: 24607953 Free PMC article. Review. - The Arf-GAP and protein scaffold Cat1/Git1 as a multifaceted regulator of cancer progression.
Yoo SM, Cerione RA, Antonyak MA. Yoo SM, et al. Small GTPases. 2020 Mar;11(2):77-85. doi: 10.1080/21541248.2017.1362496. Epub 2017 Dec 31. Small GTPases. 2020. PMID: 28981399 Free PMC article. Review. - Paxillin comes of age.
Deakin NO, Turner CE. Deakin NO, et al. J Cell Sci. 2008 Aug 1;121(Pt 15):2435-44. doi: 10.1242/jcs.018044. J Cell Sci. 2008. PMID: 18650496 Free PMC article. Review. - Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility.
Kim TY, Vigil D, Der CJ, Juliano RL. Kim TY, et al. Cancer Metastasis Rev. 2009 Jun;28(1-2):77-83. doi: 10.1007/s10555-008-9167-2. Cancer Metastasis Rev. 2009. PMID: 19221866 Free PMC article. Review.
References
- Brown, M.C., and C.E. Turner. 2004. Paxillin: adapting to change. Physiol. Rev. 84:1315–1339. - PubMed
- Cau, J., and A. Hall. 2005. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J. Cell Sci. 118:2579–2587. - PubMed
- Chew, T.L., R.A. Masaracchia, Z.M. Goeckeler, and R.B. Wysolmerski. 1998. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J. Muscle Res. Cell Motil. 19:839–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous