Re-epithelialisation and the possible involvement of the transcription factor, basonuclin - PubMed (original) (raw)
Review
Re-epithelialisation and the possible involvement of the transcription factor, basonuclin
Kyoichi Matsuzaki et al. Int Wound J. 2004 Jun.
Abstract
This article briefly summarises the basic mechanism of re-epithelialisation and discusses the possible role of the cell-type-specific transcription factor, basonuclin. Re-epithelialisation is initiated by a signal resulting from the absence of neighbouring cells at the wound edge. Basal cells at the wound edge become flattened and lose their intercellular desmosomes and substratum attachment. The amount of cytoplasmic actinomyosin filaments that insert into the new adhesion complexes is increased, and contraction of those filaments produces cell movement. The epithelial cells at the wound edge migrate on a provisional matrix using the newly expressed integrin receptors. Once re-epithelialisation is complete, the epithelial cells revert to the normal phenotype of basal epidermal cells, firmly attach to the newly developed basement membrane zone through hemidesmosomes and resume standard differentiation. Protein synthesis increases in the epidermal cells at the wound edge during re-epithelialisation. Active protein synthesis requires accelerated transcription of ribosomal RNA genes. The transcription factor basonuclin binds to the ribosomal RNA gene promoter and increases the transcription of the genes. Therefore, it is speculated that basonuclin in epithelial cells is required in the process of re-epithelialisation.
Figures
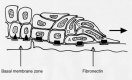
Figure 1
Cartoon to illustrate epithelial cell migration by leapfrog movement and modulation of basal cell attachment. This cartoon is a modification of Grinnell's illustration (6) and Krawczyk's diagrammatic representation (10). Migrating front A cell is overtaken by B cell. C cell is climbing over the newly adherent basal cell B. C cell will be replaced in turn by a cell D. Hemidesmosomes (triangles) normally bind to basal cells and to laminin in the basal lamina of basement membrane zone (BMZ). The epidermal cells migrate on the provisional wound matrix — fibronectin using the newly expressed α5β1 integrin receptors (squares) instead of hemidesmosomes.
Figure 2
Translocation of basonuclin from cytoplasm to nucleus (Bar = 50 µm). (A) Basonuclin (red) of the plantar epidermis of the rat is confined to the basal layer. Basonuclin is located mainly in cytoplasm. (B) Nuclear Hoechst 33258 (blue) staining does not overlap with cytoplasmic basonuclin. (C) Six‐day colonies in a secondary culture epithelia derived from plantar epidermis of the rat. Basonuclin is concentrated in the cell nuclei. (D) Nuclear Hoechst 33258 staining overlaps with nuclear basonuclin.
Similar articles
- Spreading, proliferation, and differentiation of the epidermis after wounding a cichlid fish, Hemichromis bimaculatus.
Quilhac A, Sire JY. Quilhac A, et al. Anat Rec. 1999 Mar;254(3):435-51. doi: 10.1002/(SICI)1097-0185(19990301)254:3<435::AID-AR15>3.0.CO;2-D. Anat Rec. 1999. PMID: 10096676 - Basonuclin-null mutation impairs homeostasis and wound repair in mouse corneal epithelium.
Zhang X, Tseng H. Zhang X, et al. PLoS One. 2007 Oct 31;2(10):e1087. doi: 10.1371/journal.pone.0001087. PLoS One. 2007. PMID: 17971852 Free PMC article. - Influence of acidic pH on keratinocyte function and re-epithelialisation of human in vitro wounds.
Lönnqvist S, Emanuelsson P, Kratz G. Lönnqvist S, et al. J Plast Surg Hand Surg. 2015;49(6):346-52. doi: 10.3109/2000656X.2015.1053397. Epub 2015 Jun 7. J Plast Surg Hand Surg. 2015. PMID: 26051107 - The epithelialisation phase in wound healing: options to enhance wound closure.
Tomic-Canic M, Wong LL, Smola H. Tomic-Canic M, et al. J Wound Care. 2018 Oct 2;27(10):646-658. doi: 10.12968/jowc.2018.27.10.646. J Wound Care. 2018. PMID: 30332358 Review. - Physiology of the acute wound.
Lawrence WT. Lawrence WT. Clin Plast Surg. 1998 Jul;25(3):321-40. Clin Plast Surg. 1998. PMID: 9696896 Review.
Cited by
- The human airway epithelial basal cell transcriptome.
Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. Hackett NR, et al. PLoS One. 2011 May 4;6(5):e18378. doi: 10.1371/journal.pone.0018378. PLoS One. 2011. PMID: 21572528 Free PMC article. - Proteomic Changes during the Dermal Toxicity Induced by Nemopilema nomurai Jellyfish Venom in HaCaT Human Keratinocyte.
Choudhary I, Hwang D, Chae J, Yoon W, Kang C, Kim E. Choudhary I, et al. Toxins (Basel). 2021 Apr 27;13(5):311. doi: 10.3390/toxins13050311. Toxins (Basel). 2021. PMID: 33925349 Free PMC article. - Induction of neuro-protective/regenerative genes in stem cells infiltrating post-ischemic brain tissue.
Yilmaz G, Alexander JS, Erkuran Yilmaz C, Granger DN. Yilmaz G, et al. Exp Transl Stroke Med. 2010 May 28;2(1):11. doi: 10.1186/2040-7378-2-11. Exp Transl Stroke Med. 2010. PMID: 20509949 Free PMC article.
References
- Stenn KS, Malhotra R. Epithelialization. In: Cohen IK, Diegelmann RF, Lindblad WJ, editors. Wound healing: biochemical and clinical aspects. Philadelphia: Saunders, 1992: 115–27.
- Tseng H, Biegel JA, Brown RS. Basonuclin is associated with the ribosomal RNA genes on human keratinocyte mitotic chromosomes. J Cell Sci 1999; 112: 3039–47. - PubMed
- Clark RAF. Cutaneous tissue repair: basic biologic considerations. J Am Acad Dermatol 1985;13: 701–25. - PubMed
- Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med 1999;341: 738–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical