Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells - PubMed (original) (raw)
Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells
Laurence Catley et al. Blood. 2006.
Abstract
Histone deacetylase (HDAC) inhibitors have shown cytotoxicity as single agents in preclinical studies for multiple myeloma (MM) cells. LBH589 is a novel hydroxamic acid derivative that at low nanomolar concentrations induces apoptosis in MM cells resistant to conventional therapies via caspase activation and poly-(ADP-ribose) polymerase (PARP) cleavage. Significant synergistic cytotoxicity was observed with LBH589 in combination with bortezomib against MM cells that were sensitive and resistant to dexamethasone (Dex), as well as primary patient MM cells. LBH589 at low nanomolar concentrations also induced alpha-tubulin hyperacetylation. Aggresome formation was observed in the presence of bortezomib, and the combination of LBH589 plus bortezomib induced the formation of abnormal bundles of hyeracetylated alpha-tubulin but with diminished aggresome size and apoptotic nuclei. These data confirm the potential clinical benefit of combining HDAC inhibitors with proteasome inhibitors, and provide insight into the mechanisms of synergistic anti-MM activity of bortezomib in combination with LBH589.
Figures
Figure 1.
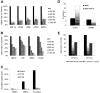
LBH589 induces cytotoxicity in human MM cell lines resistant to conventional chemotherapy. (A) Viability of MM.1S, RPMI8226, U266, OPM1, and KMS11 at 48 hours was inhibited by LBH589 in a dose-dependent manner as demonstrated by MTS assay. (B) Growth of Dex-resistant MM.1R cells and RPMI8226 MM cells resistant to Dox (Dox40 cells), Mit (MR20 cells), or Mel (LR5 cells) was inhibited in a dose-dependent manner, as demonstrated by MTS assay. (C) Adhesion of MM.1S cells to BMSCs induced a significant increase in 3H-thymidine uptake by MM.1S cells at 48 hours, which was completely inhibited by LBH589. Supernatants from the experiment (C) were examined for cytokine secretion (D). LBH589 inhibited adhesion-induced up-regulation of IL-6 secretion in BMSCs triggered by coculture with MM.1S. (E) LBH589 up to 1 μM decreased BMSC viability, with 61% viability at 48 hours and 59% viability at 72 hours. Data represent mean ± SD of quadruplicate cultures.
Figure 2.
LBH589 potently induces histone and α-tubulin hyperacetylation as well as apoptotic signaling. MM.1S cells were incubated with up to 100 nM LBH589 for 24 hours, and whole-cell extracts were analyzed by Western blot. (A) There was a dose-dependent increase in both histone acetylation (top panel) and α-tubulin acetylation (bottom panel). (B) Cleavage of caspase-8, -9, -3 and PARP was induced in a dose-dependent manner. In addition, p21 was up-regulated and c-myc was down-regulated. FL indicates full length; CL, cleavage fragment.
Figure 3.
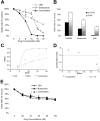
LBH589 and bortezomib trigger synergistic cytotoxicity in RPMI8226 MM cell lines. (A) RPMI8226 MM cells were incubated with LBH589 (0-20 nM), bortezomib (0-20 nM), or both for 48 hours. Synergistic cytotoxicity was demonstrated by MTS assay. (B) Cytotoxicity of LBH589 and bortezomib was partly inhibited by pan-caspase inhibitor ZVAD-FMK. Data represent mean ± SD of quadruplicate cultures. The viability, as determined by MTS assay and shown in panel A, was converted into effect (1-viability), and shown as an isobologram graph (C), as well as converted to combination index (CI) (D) according to the Chou-Talalay equation (“Materials and methods”). Synergy is present when the CI is less than 1.0. The combination is additive when CI equals 1.0, and antagonistic when it is more than 1.0. Synergy was observed with a CI of 0.404, 0.054, and 0.067 at 12, 16, and 20 nM of LBH589 and bortezomib. (E) Healthy donor peripheral-blood mononuclear cells (PBMCs) were incubated with LBH589 (0-100 nM), bortezomib (0-100 nM), or both for 48 hours. No synergy was observed against PBMCs. Data represent mean ± SD of quadruplicate cultures.
Figure 4.
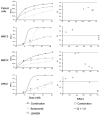
LBH589 and bortezomib induce synergistic cytotoxicity against MM cell lines sensitive and resistant to Dex, as well as freshly isolated CD138+ patient MM cells. (A-D) Synergistic cytotoxicity of LBH589 and bortezomib was observed in RPMI8226 and MM.1S, patient cells, and OPM1 (4-20 nM), as well as in MM.1R cells (10-50 nM), as determined by MTS assay and presented as a fraction of cells affected (left panels) and combination indices (right panels).
Figure 5.
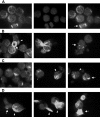
Bortezomib-induced aggresome formation and LBH589-induced α-tubulin hyperacetylation. RPMI8226 cells were treated for 24 hours with 16 nM LBH589, 16 nM bortezomib, or both, stained with anti-acetyl-α-tubulin and DAPI, then examined by inverted microscopy. (A) In untreated RPMI8226 cells, acetylated α-tubulin showed a reticulated appearance (left panel). The center panel shows the DAPI stain. In the merged image in the right panel, a normal mitotic figure (arrow, right panel) is shown, as well as other nondividing cells. (B) HDAC inhibitor LBH589 induced diffuse or segmented α-tubulin hyperacetylation. Hyperacetylated-α-tubulin (arrow, left panel); diffuse acetyl-α-tubulin (arrowhead, left panel); segmented α-tubulin hyperacetylation (center panel); mitotic figure (arrow, right panel). (C) Proteasome inhibitor bortezomib induced aggresomes in the presence of normal reticulated α-tubulin (left panel). Focal aggresome (arrow, right panel); apoptotic nuclei (arrowheads, center and right panels). (D) LBH589 and bortezomib induced large bundles of hyperacetylated α-tubulin (arrowheads, left and center panels) that were not seen in cells treated with LBH589 alone. Diffuse hyperacetylated α-tubulin was also observed (arrow, right panel). Although aggresome formation was observed (arrow, left panel), here they were smaller than when treated with bortezomib alone, and were associated with a diffuse cytoplasmic background and apoptotic nuclei.
Figure 6.
Bortezomib-induced aggresome formation and LBH589-induced α-tubulin hyperacetylation are augmented in combination. Patterns of α-tubulin acetylation and aggresome formation were classified as described in “Materials and methods.” Cell counts were performed on at least 150 cells in each treatment group. Morphologic patterns defined by acetyated α-tubulin were further classified based on nuclear appearance to be either normal or apoptotic. A representative set of counts from 3 separate experiments is shown. Data represent means ± SD.
Figure 7.
Schematic representation of upstream cytoskeletal events when bortezomib and LBH589 are combined. (A) Viable plasma cell with reticulated acetylated α-tubulin. (B) Aggresome formation in the presence of bortezomib. Aggregates of misfolded proteins are directed along the acetylated α-tubulin fibrils toward a single perinuclear region, forming the aggresome. This process requires TDAC activity. (C) In the presence of bortezomib in combination with LBH589, aggresome formation is diminished, bundles of hyperacetylated α-tubulin form, and the cell undergoes apoptosis.
Similar articles
- [Study on histone deacetylase inhibitor LBH589 induces apoptosis of multiple myeloma cells and its reversal of drug resistance mechanism].
Zhang L, Ma YP, Jia G. Zhang L, et al. Zhonghua Xue Ye Xue Za Zhi. 2012 Nov;33(11):926-31. Zhonghua Xue Ye Xue Za Zhi. 2012. PMID: 23363750 Chinese. - Antiproliferative and proapoptotic effects of proteasome inhibitors and their combination with histone deacetylase inhibitors on leukemia cells.
Fuchs O, Provaznikova D, Marinov I, Kuzelova K, Spicka I. Fuchs O, et al. Cardiovasc Hematol Disord Drug Targets. 2009 Mar;9(1):62-77. doi: 10.2174/187152909787581372. Cardiovasc Hematol Disord Drug Targets. 2009. PMID: 19275578 Review. - Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma.
Hideshima T, Richardson PG, Anderson KC. Hideshima T, et al. Mol Cancer Ther. 2011 Nov;10(11):2034-42. doi: 10.1158/1535-7163.MCT-11-0433. Mol Cancer Ther. 2011. PMID: 22072815 Free PMC article. Review.
Cited by
- When Cancer Fights Back: Multiple Myeloma, Proteasome Inhibition, and the Heat-Shock Response.
Shah SP, Lonial S, Boise LH. Shah SP, et al. Mol Cancer Res. 2015 Aug;13(8):1163-73. doi: 10.1158/1541-7786.MCR-15-0135. Epub 2015 May 26. Mol Cancer Res. 2015. PMID: 26013169 Free PMC article. Review. - Emerging Therapeutic Strategies to Overcome Drug Resistance in Multiple Myeloma.
Davis LN, Sherbenou DW. Davis LN, et al. Cancers (Basel). 2021 Apr 2;13(7):1686. doi: 10.3390/cancers13071686. Cancers (Basel). 2021. PMID: 33918370 Free PMC article. Review. - Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat.
Tate CR, Rhodes LV, Segar HC, Driver JL, Pounder FN, Burow ME, Collins-Burow BM. Tate CR, et al. Breast Cancer Res. 2012 May 21;14(3):R79. doi: 10.1186/bcr3192. Breast Cancer Res. 2012. PMID: 22613095 Free PMC article. - Panobinostat (Farydak): A Novel Option for the Treatment of Relapsed Or Relapsed and Refractory Multiple Myeloma.
Moore D. Moore D. P T. 2016 May;41(5):296-300. P T. 2016. PMID: 27162469 Free PMC article. No abstract available. - The Role of Epigenetics in the Development and Progression of Multiple Myeloma.
Ismail NH, Mussa A, Zakaria NA, Al-Khreisat MJ, Zahidin MA, Ramli NN, Mohammad SNNA, Hassan R, Mohd Noor NH, Iberahim S, Zulkafli Z, Mohamed Yusoff S, Husin A, Johan MF. Ismail NH, et al. Biomedicines. 2022 Oct 31;10(11):2767. doi: 10.3390/biomedicines10112767. Biomedicines. 2022. PMID: 36359286 Free PMC article. Review.
References
- Remiszewski SW. Recent advances in the discovery of small molecule histone deacetylase inhibitors. Curr Opin Drug Discov Devel. 2002;5: 487-499. - PubMed
- Remiszewski SW. The discovery of NVP-LAQ824: from concept to clinic. Curr Med Chem. 2003;10: 2393-2402. - PubMed
- Marks PA, Rifkind RA, Richon VM. Histone deacetylase inhibitors: development of suberoylanilide hydroxamic acid (SAHA) for the treatment of cancers. Proc Natl Acad Sci U S A. 2001;98: 10572-10577. - PubMed
- Mitsiades N, Mitsiades CS, Richardson PG, et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood. 2003;101: 4055-4062. - PubMed
- Catley L, Weisberg E, Tai YT, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102: 2615-2622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical