Bovine herpesvirus 1 UL49.5 protein inhibits the transporter associated with antigen processing despite complex formation with glycoprotein M - PubMed (original) (raw)
Bovine herpesvirus 1 UL49.5 protein inhibits the transporter associated with antigen processing despite complex formation with glycoprotein M
Andrea D Lipińska et al. J Virol. 2006 Jun.
Abstract
Bovine herpesvirus 1 (BHV-1) interferes with peptide translocation by the transporter associated with antigen processing (TAP). Recently, the UL49.5 gene product of BHV-1 was identified as the protein responsible for the observed inhibition of TAP. In BHV-1-infected cells and virions, the UL49.5 protein forms a complex with glycoprotein M (gM). Hence, it was investigated whether UL49.5 can combine the interactions with gM and the TAP complex. In cell lines constitutively expressing both UL49.5 and gM, UL49.5 appears to be required for functional processing of gM. Immunofluorescence-confocal laser scanning microscopy demonstrated that both proteins are interdependent for their redistribution from the endoplasmic reticulum to the trans-Golgi network. Remarkably, expression of cloned gM results in the abrogation of the UL49.5-mediated inhibition of TAP and prevents the degradation of the transporter. However, in BHV-1-infected cells, differences in UL49.5 and gM expression kinetics were seen to create a window of opportunity at the early stages of infection, during which time the UL49.5 protein can act on TAP without gM interference. Moreover, in later periods, non-gM-associated UL49.5 can be detected in addition to the UL49.5/gM complex. Thus, it has been deduced that different functions of UL49.5, editing of gM processing and inhibition of TAP, can be combined during BHV-1 infection.
Figures
FIG. 1.
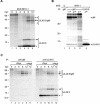
UL49.5 is required for gM maturation. (A) Cell lysates of control MJS (c), BHV-1-infected MJS, and MJS stably expressing gM or UL49.5/gM were treated with endoglycosidase H (EndoH) (+) or left untreated (−). The EndoH-resistant forms (gM EndoH R), the EndoH-sensitive form (gM EndoH S), and the peptide:_N_-glycosidase F (PNGaseF)-treated, deglycosylated form of gM (gM-CHO) were detected by immunoblotting with gM-specific antibodies. (B) UL49.5 and gM form a complex in MJS UL49.5/gM cells. UL49.5 or gM was immunoprecipitated (IP) with specific rabbit antibodies from control (c) or UL49.5/gM-coexpressing cells. Immunoprecipitates were separated by SDS-PAGE and stained by immunoblotting (WB) with mouse antibodies against either gM (upper panel) or UL49.5. (C) The transmembrane region of UL49.5 is important for functional processing of gM. The mature gM (solid circle) could be detected by immunoblotting either in full-length UL49.5/gM-coexpressing or tailless UL49.5/gM-coexpressing MJS (MJS UL49.5 Δtail/gM). Coexpression of UL49.5 lacking both its tail and transmembrane region in MJS UL49.5 Δtail ΔTM/gM cells resulted in impaired maturation of high-mannose gM (open circle), comparable to MJS gM-expressing cells. The 34-kDa gM precursor is indicated with a dashed circle. c, control MJS; *, immunoglobulin heavy chain. Size markers are in kilodaltons.
FIG. 2.
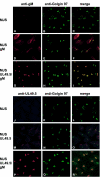
Subcellular redistribution of UL49.5/gM analyzed by confocal laser scanning microscopy. gM was detected with gM-specific rabbit antibodies in MJS (A and C), MJS gM (D and F), or MJS UL49.5/gM (G and I). UL49.5 was detected with UL49.5-specific rabbit antibodies in MJS (J and L), MJS UL49.5 (M and O), or MJS UL49.5/gM (P and R). The _trans_-Golgi compartment was stained with MAb anti-Golgin 97 (B, E, H, K, N, and Q). The anti-gM and anti-UL49.5 rabbit antibodies were visualized with Alexa 594-conjugated goat anti-rabbit IgG. Golgin 97 was visualized with Alexa 633-conjugated goat anti-mouse IgG, and the signal was electronically converted into green. In the overlays, colocalization is shown in yellow.
FIG. 3.
gM interferes with UL49.5-mediated inactivation of TAP. (A) UL49.5 inhibition of TAP-dependent peptide transport is not observed in MJS UL49.5/gM cells. The translocation of fluorescent peptides in MJS UL49.5 or MJS UL49.5/gM cells is represented as a percentage of the translocation in control (c) MJS cells. FL, fluorescein. (B) UL49.5/gM coexpression results in the stabilization of TAP1 and TAP2 protein levels. Steady-state levels of TAP1, TAP2, and beta-actin were evaluated by immunoblotting in control (c) MJS, MJS UL49.5, MJS gM, or MJS UL49.5/gM cells using specific antibodies; *, unidentified background protein. (C) Downregulation of MHC class I surface expression by UL49.5 in the absence (upper panel, boldface line, no. 2) or in the presence of gM (lower panel, boldface line, no. 3). Thin line, MHC I in MJS cells (no. 1); dashed line, goat anti-mouse phycoerythrin control (c). (D) Immunoblot analysis of UL49.5 expression in MJS cells expressing UL49.5 or coexpressing UL49.5 and gM. Uninfected and BHV-1-infected MJS cells were included as controls.
FIG. 4.
UL49.5 and gM are expressed with different kinetics during BHV-1 infection. MJS cells were infected with BHV-1 in the absence or presence of phosphonoacetic acid (−PAA and +PAA, respectively), collected at the indicated time points postinfection (hpi), and metabolically labeled for 35 min. (A) UL49.5 (left panel) or gM (right panel) was immunoprecipitated from cell lysates with anti-UL49.5 and anti-gM antibodies, respectively. The fully glycosylated gM (solid circle), the high-mannose gM (open circle), and the gM precursor (dashed circle) are indicated. (B) The early glycoprotein B or the late virion host shutoff (vhs) protein were immunoprecipitated from the cell lysates as controls. (C) The experiment is the same as that described for panel A, performed in MDBK cells. (D) The early gB, the late gC, and the late vhs protein immunoprecipitated from the MDBK cell lysates. Size markers are in kilodaltons.
FIG. 5.
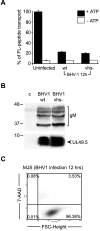
BHV-1 inhibits ATP-dependent peptide transport by TAP at late stages of infection. MJS cells were infected with wild-type (wt) BHV-1 or a virion host shutoff deletion (vhs−) mutant for 12 h. (A) TAP-dependent peptide transport was assessed in the presence or absence of ATP using a fluorescein (FL)-tagged peptide. Uninfected MJS cells were used as a control. FL-peptide translocation in infected cells is represented as a percentage of the translocation in uninfected cells (set as 100%). (B) gM and UL49.5 detected in the BHV-1-infected cells used in the TAP transport assays shown in panel A. The cell lysates were separated by SDS-PAGE and analyzed by immunoblotting using antibodies against gM (upper panel) or UL49.5 (lower panel). Size markers are in kilodaltons. (C) The viability of the BHV-1-infected cells was evaluated at 12 hpi by 7-AAD staining and flow cytometry. FSC, forward scatter.
FIG. 6.
UL49.5 is present in excess during BHV-1 infection. (A) BHV-1-infected MJS cells were collected at the indicated time points postinfection and metabolically labeled for 35 min. Cell lysates were subjected to immunoprecipitation with anti-UL49.5 antibodies and analyzed using nonreducing SDS-PAGE. Monomeric UL49.5 (solid arrow), UL49.5 dimers (double solid arrow), fully glycosylated gM-UL49.5 heterodimers (solid circle, solid arrow), high-mannose gM-UL49.5 heterodimers (open circle, solid arrow), and precursor gM-UL49.5 heterodimers (dashed circle, solid arrow) are indicated. (B) Mock-infected control cells (c) or BHV-1-infected cells were collected at 8.5 hpi and metabolically labeled for 30 min. Cell lysates were subjected to three sequential rounds of immunoprecipitation (IP) with anti-gM antibodies. After the third IP with gM, the lysate was subjected to a fourth, final IP with anti-UL49.5. (C) BHV-1-infected MDBK cells were collected at the indicated time points postinfection and metabolically labeled for 35 min. Cell lysates were subjected to immunoprecipitation with anti-gM (left panel) or anti-UL49.5 (right panel) antibodies and analyzed using nonreducing SDS-PAGE. Monomeric UL49.5, UL49.5 dimers, fully glycosylated gM-UL49.5 heterodimers, high-mannose gM-UL49.5 heterodimers, and precursor gM-UL49.5 heterodimers are indicated as for panel A. Size markers are in kilodaltons.
FIG. 7.
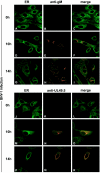
Subcellular redistribution of UL49.5 and gM in BHV-1-infected MDBK cells, analyzed by confocal laser scanning microscopy. MDBK cells were infected with BHV-1 at an MOI of 1. gM was detected in infected cells with gM-specific rabbit antibodies at the indicated times postinfection (B, C, E, F, H, and I). UL49.5 was detected with UL49.5-specific rabbit antibodies (K, L, N, O, Q, and R). The ER/_cis_-Golgi was stained with concanavalin A-Alexa 488 (A, C, D, F, G, I, J, L, M, O, P, and R). The anti-gM and anti-UL49.5 rabbit antibodies were visualized with Alexa 594-conjugated goat anti-rabbit IgG. In the overlays, colocalization is shown in yellow.
Similar articles
- Transmembrane regions of bovine herpesvirus 1-encoded UL49.5 and glycoprotein M regulate complex maturation and ER-Golgi trafficking.
Graul M, Kisielnicka E, Rychłowski M, Verweij MC, Tobler K, Ackermann M, Wiertz EJHJ, Bieńkowska-Szewczyk K, Lipińska AD. Graul M, et al. J Gen Virol. 2019 Mar;100(3):497-510. doi: 10.1099/jgv.0.001224. Epub 2019 Jan 29. J Gen Virol. 2019. PMID: 30694168 - Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing.
Koppers-Lalic D, Reits EA, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes Rezende M, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, Mettenleiter TC, Rijsewijk FA, Tampé R, Neefjes J, Wiertz EJ. Koppers-Lalic D, et al. Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5144-9. doi: 10.1073/pnas.0501463102. Epub 2005 Mar 25. Proc Natl Acad Sci U S A. 2005. PMID: 15793001 Free PMC article. - Bovine Herpesvirus 1 UL49.5 Interacts with gM and VP22 To Ensure Virus Cell-to-Cell Spread and Virion Incorporation: Novel Role for VP22 in gM-Independent UL49.5 Virion Incorporation.
Pannhorst K, Wei H, Yezid H, He J, Chowdhury SI. Pannhorst K, et al. J Virol. 2018 Jun 13;92(13):e00240-18. doi: 10.1128/JVI.00240-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29669828 Free PMC article. - Why does the herpes simplex 1 virus-encoded UL49.5 protein fail to inhibit the TAP-dependent antigen presentation?
Karska N, Zhukov I, Lipińska AD, Rodziewicz-Motowidło S, Krupa P. Karska N, et al. Biochim Biophys Acta Biomembr. 2023 Dec;1865(8):184200. doi: 10.1016/j.bbamem.2023.184200. Epub 2023 Jul 29. Biochim Biophys Acta Biomembr. 2023. PMID: 37517559 Review. - A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines.
Jones C, Chowdhury S. Jones C, et al. Anim Health Res Rev. 2007 Dec;8(2):187-205. doi: 10.1017/S146625230700134X. Anim Health Res Rev. 2007. PMID: 18218160 Review.
Cited by
- Viral inhibition of the transporter associated with antigen processing (TAP): a striking example of functional convergent evolution.
Verweij MC, Horst D, Griffin BD, Luteijn RD, Davison AJ, Ressing ME, Wiertz EJ. Verweij MC, et al. PLoS Pathog. 2015 Apr 16;11(4):e1004743. doi: 10.1371/journal.ppat.1004743. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25880312 Free PMC article. Review. - The Roles of Envelope Glycoprotein M in the Life Cycle of Some Alphaherpesviruses.
Li C, Wang M, Cheng A, Jia R, Yang Q, Wu Y, Zhu D, Zhao X, Chen S, Liu M, Zhang S, Ou X, Mao S, Gao Q, Sun D, Wen X, Tian B. Li C, et al. Front Microbiol. 2021 Feb 19;12:631523. doi: 10.3389/fmicb.2021.631523. eCollection 2021. Front Microbiol. 2021. PMID: 33679658 Free PMC article. Review. - Targeted degradation of ABC transporters in health and disease.
Nikles D, Tampé R. Nikles D, et al. J Bioenerg Biomembr. 2007 Dec;39(5-6):489-97. doi: 10.1007/s10863-007-9120-z. J Bioenerg Biomembr. 2007. PMID: 17972020 Review. - Structural and Functional Dissection of the Human Cytomegalovirus Immune Evasion Protein US6.
Dugan GE, Hewitt EW. Dugan GE, et al. J Virol. 2008 Apr;82(7):3271-82. doi: 10.1128/JVI.01705-07. Epub 2008 Jan 16. J Virol. 2008. PMID: 18199642 Free PMC article. - Alphaherpesvirus gB Homologs Are Targeted to Extracellular Vesicles, but They Differentially Affect MHC Class II Molecules.
Grabowska K, Wąchalska M, Graul M, Rychłowski M, Bieńkowska-Szewczyk K, Lipińska AD. Grabowska K, et al. Viruses. 2020 Apr 10;12(4):429. doi: 10.3390/v12040429. Viruses. 2020. PMID: 32290097 Free PMC article.
References
- Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. - PubMed
- Beinert, D., L. Neumann, S. Uebel, and R. Tampe. 1997. Structure of the viral TAP-inhibitor ICP47 induced by membrane association. Biochemistry 36:4694-4700. - PubMed
- Boname, G. M., B. D. de Lima, P. J. Lehner, and P. G. Stevenson. 2004. Viral degradation of the MHC class I peptide loading complex. Immunity 20:305-317. - PubMed
- Boname, G. M., and P. G. Stevenson. 2001. MHC class I ubiquitination by a viral PHD/LAP finger protein. Immunity 15:627-636. - PubMed
- Cresswell, P. 2000. Intracellular surveillance: controlling the assembly of MHC class I-peptide complexes. Traffic 1:301-305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous