Cellular and biochemical differences between two attenuated poxvirus vaccine candidates (MVA and NYVAC) and role of the C7L gene - PubMed (original) (raw)
Comparative Study
Cellular and biochemical differences between two attenuated poxvirus vaccine candidates (MVA and NYVAC) and role of the C7L gene
José Luis Nájera et al. J Virol. 2006 Jun.
Abstract
The poxvirus strains NYVAC and MVA are two candidate vectors for the development of vaccines against a broad spectrum of diseases. Although these attenuated virus strains have proven to be safe in animals and humans, little is known about their comparative behavior in vitro. In contrast with MVA, NYVAC infection triggers greater cytopathic effect in a range of permissive and nonpermissive cell lines. The yields of NYVAC cell-associated virus in permissive cells (BHK-21) were slightly reduced compared with those of MVA infection. During the course of infection in HeLa cells, there is a translational block induced by NYVAC late in infection, which correlated with a marked increase in phosphorylation levels of the initiation factor eIF-2alpha. In contrast to MVA, the synthesis of certain late viral proteins was only blocked in NYVAC-infected HeLa cells. Electron microscopy (EM) analysis revealed that morphogenesis of NYVAC in HeLa cells was blocked at the stage of formation of immature viral forms. Phase-contrast microscopy, EM, flow cytometry, and rRNA analyses demonstrated that contrary to MVA, NYVAC infection induces potent apoptosis, a phenomenon dependent on activation of caspases and RNase L. Apoptosis induced by NYVAC was prevented when the virus gene C7L was placed back into the NYVAC genome, recovering the ability of NYVAC to replicate in HeLa cells and maintaining the attenuated phenotype in mice. Overall, our findings demonstrate distinct behavior between NYVAC and MVA strains in cultured cells, as well as a new role for the C7L viral gene as an inhibitor of apoptosis in NYVAC infection.
Figures
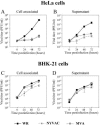
FIG. 1.
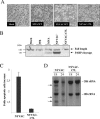
CPE of MVA and NYVAC in human cells. Monolayers of HeLa cells were mock infected or infected at 5 PFU/cell with WR, MVA, or NYVAC. At different times postinfection, as indicated in the figure, the morphological changes in the cells were examined by phase-contrast microscopy. Mock, uninfected cells.
FIG. 2.
Virus growth of MVA and NYVAC in permissive and nonpermissive cell lines. Monolayers of HeLa and BHK-21 cells were infected at 0.01 PFU/cell with WR, MVA, or NYVAC for 0, 24, 48, and 72 h. Cells were collected by centrifugation, and infectious virus associated with the cells (A and C) and released to the supernatant (B and D) during the course of the infection was quantified by immunostaining assay. For comparative purposes, we used the replication-competent WR strain. Averages of three independent experiments are shown with standard error bars.
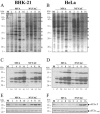
FIG. 3.
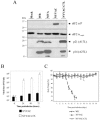
Protein synthesis during NYVAC and MVA infection. Monolayers of BHK-21 (A) and HeLa (B) cells were mock infected (M) or infected at 5 PFU/cell with MVA or NYVAC. At the indicated times (h p.i.), cells were metabolically labeled for 30 min with [35S]Met-Cys Promix (50 μCi/ml) and equal amounts of proteins were analyzed by SDS-PAGE (10%) and autoradiography. The dots on the right indicate prominent viral proteins. (C and D) Western blot showing expression of VV antigens during the time course of MVA and NYVAC infection in infected BHK-21 (C) and HeLa (D) cells. The blot was probed with a rabbit polyclonal antiserum (1:500 dilution) raised against live VV. Numbers appearing under each lane represent the ratio of intensity of the bands in infected cells to levels in uninfected cells, as determined by densitometric analyses. (E and F) Western blot analysis of total eIF2-α and phospho-eIF-2α-S51 protein levels during the time course of MVA and NYVAC infection in infected BHK-21 (E) and HeLa (F) cells.
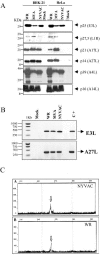
FIG. 4.
Expression of specific viral proteins under permissive and nonpermissive conditions. (A) Monolayers of BHK-21 and HeLa cells were mock infected (M) or infected at 5 PFU/cell with WR, MVA, or NYVAC, and cell extracts were analyzed by Western blotting. Cell lysates were harvested at 24 h p.i., and equal amounts of proteins were fractionated by SDS-PAGE, transferred to nitrocellulose paper, and reacted with different antibodies recognizing specific viral early proteins, such as p25, and viral late proteins, such as p27.5, p21, p14, p39, or p16. (B) Transcription of early and late viral genes. The transcription of E3L and A27L genes was determined by RT-PCR from total RNAs as described in Materials and Methods. Total RNA from uninfected cells and DNA extracted from MVA-infected cells were used as the negative (Mock) and positive (C+) control, respectively. (C) Primer extension product obtained using 2 μg of total RNA isolated from HeLa cells either uninfected (Mock) or infected at 5 PFU/cell with WR or NYVAC for 16 h. The sizes of the peaks from the GeneScan-500 ROX internal lane standards are shown (in base pairs). The arrows indicate the primer extension products (VIC-labeled cDNA) for the A27L gene. Peak height is a measure of fluorescence intensity and indicates the strength of the VIC signal. The peak heights for each sample were 177 for NYVAC (A), 164 for WR (B), and 55 for mock infected (not shown).
FIG. 5.
Electron microscopy of NYVAC morphogenesis in HeLa cells. (A and B) Electron micrographs of HeLa cells infected with 5 PFU/cell of NYVAC (A) or with 5 PFU/cell of MVA (B) at 16 h p.i. The magnification of each panel is indicated by bars in the upper right corner. Characteristics of apoptosis in NYVAC-infected cells are shown in panels C to E. (C) An infected cell with nuclear condensation (white asterisk). (D) Cytoplasm of an infected cell with extensive vacuolation (black asterisks). (E) Cytoplasm of an infected cell with dense mitochondria (arrowheads). Bars = 500 nm.
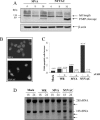
FIG. 6.
Infection with NYVAC induces apoptosis in nonpermissive HeLa cells. (A) Western blot analysis of PARP cleavage in HeLa cells infected with 5 PFU/cell of MVA or NYVAC at different times postinfection. (B) DAPI staining of HeLa cells infected with 5 PFU/cell of MVA and NYVAC at 24 h p.i. (C) Monolayers of HeLa cells were infected at 5 PFU/cell with WR, MVA, or NYVAC in the presence or absence of zVAD (40 μM). At 24 h p.i., the infected cells were stained with propidium iodide followed by cell cycle analysis using a flow cytometer to detect cells with hypodiploid DNA content. Untreated HeLa cells were used as a negative control (Mock). Bars represent the fold increase in apoptotic cells with respect to mock infected. The percentage of apoptotic cells is indicated over the bars. Similar results were obtained in two independent experiments. (D) rRNA breakdown. HeLa cells were mock infected or infected with WR, MVA, or NYVAC at 5 PFU/cell. Total RNA was isolated at 18 and 24 h p.i., and 2 μg of each was applied for electrophoresis. The arrows indicate bands corresponding to characteristic degradation products of rRNA.
FIG. 7.
The apoptotic phenotype of NYVAC is inhibited by the expression of the virus C7L host range gene. Monolayers of HeLa cells were infected with recombinant NYVAC-C7L or with MVA or the NYVAC wild type (WT) at 5 PFU/cell. (A) The morphology of cells in apoptosis was observed by phase-contrast microscopy. (B) Western blot analysis of PARP cleavage in HeLa cells infected with WR, MVA, NYVAC, or NYVAC-C7L at 24 h p.i. (C) Monolayers of HeLa cells were infected at 5 PFU/cell with NYVAC or NYVAC-C7L. At 24 h p.i., the infected cells were stained with propidium iodide followed by cell cycle analysis using a flow cytometer to detect cells with hypodiploid DNA. Uninfected HeLa cells (Mock) were used as a negative control. Bars represent the fold increase in apoptotic cells with respect to mock infected. (D) rRNA breakdown. HeLa cells were infected with NYVAC or NYVAC-C7L at 5 PFU/cell. Total RNA was isolated at 18 and 24 h p.i., and 2 μg of each was applied for electrophoresis. The arrows indicate bands corresponding to characteristic degradation products of rRNA.
FIG. 8.
The VV C7L gene rescued NYVAC translation capacity and virus growth in human cells but retained the attenuated phenotype in vivo. (A) Expression of total and phospho-eIF2-α and late viral proteins (p21 and p14) in HeLa cells uninfected (Mock) or infected with 5 PFU/cell of WR, MVA, NYVAC, or NYVAC-C7L at 24 h p.i. was analyzed by Western blotting. (B) Monolayers of HeLa cells were infected at 0.01 PFU/cell with NYVAC or NYVAC-C7L virus for 0, 24, 48, and 72 h. Cells were collected by centrifugation, and infectious virus associated with the cells during the course of the infection was quantified by immunostaining assay. Two independent experiments are shown with standard error bars. (C) BALB/c mice (n = 4) were inoculated by the i.n. route with different challenge doses of either NYVAC or NYVAC-C7L (from 106 to 108 PFU/mouse) or with 106 PFU/mouse of WR. Body weight was monitored daily and is expressed as the mean for each group. Animals suffering from severe systemic infection and having lost >25% body weight were sacrificed (black cross). The graph represents the values obtained using the highest dose of NYVAC and NYVAC-C7L (108 PFU/mouse) and the low dose (106 PFU/mouse) of WR.
FIG. 9.
Scheme of deleted genes in MVA and NYVAC genomes. Genome maps of MVA and NYVAC strains adapted from Antoine et al. (1) are represented. The deleted or fragmented genes in each genome are indicated. The right and left terminal regions are shown.
Similar articles
- Head-to-head comparison on the immunogenicity of two HIV/AIDS vaccine candidates based on the attenuated poxvirus strains MVA and NYVAC co-expressing in a single locus the HIV-1BX08 gp120 and HIV-1(IIIB) Gag-Pol-Nef proteins of clade B.
Gómez CE, Nájera JL, Jiménez EP, Jiménez V, Wagner R, Graf M, Frachette MJ, Liljeström P, Pantaleo G, Esteban M. Gómez CE, et al. Vaccine. 2007 Apr 12;25(15):2863-85. doi: 10.1016/j.vaccine.2006.09.090. Epub 2006 Oct 16. Vaccine. 2007. PMID: 17113200 - Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC.
Guerra S, Nájera JL, González JM, López-Fernández LA, Climent N, Gatell JM, Gallart T, Esteban M. Guerra S, et al. J Virol. 2007 Aug;81(16):8707-21. doi: 10.1128/JVI.00444-07. Epub 2007 May 30. J Virol. 2007. PMID: 17537851 Free PMC article. - Generation and immunogenicity of novel HIV/AIDS vaccine candidates targeting HIV-1 Env/Gag-Pol-Nef antigens of clade C.
Gómez CE, Nájera JL, Jiménez V, Bieler K, Wild J, Kostic L, Heidari S, Chen M, Frachette MJ, Pantaleo G, Wolf H, Liljeström P, Wagner R, Esteban M. Gómez CE, et al. Vaccine. 2007 Mar 1;25(11):1969-92. doi: 10.1016/j.vaccine.2006.11.051. Epub 2006 Dec 6. Vaccine. 2007. PMID: 17224219 - Highly attenuated poxvirus vectors: NYVAC, ALVAC and TROVAC.
Paoletti E, Taylor J, Meignier B, Meric C, Tartaglia J. Paoletti E, et al. Dev Biol Stand. 1995;84:159-63. Dev Biol Stand. 1995. PMID: 7796949 Review. - The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer.
Gómez CE, Nájera JL, Krupa M, Esteban M. Gómez CE, et al. Curr Gene Ther. 2008 Apr;8(2):97-120. doi: 10.2174/156652308784049363. Curr Gene Ther. 2008. PMID: 18393831 Review.
Cited by
- Improving Adaptive and Memory Immune Responses of an HIV/AIDS Vaccine Candidate MVA-B by Deletion of Vaccinia Virus Genes (C6L and K7R) Blocking Interferon Signaling Pathways.
García-Arriaza J, Arnáez P, Gómez CE, Sorzano CÓ, Esteban M. García-Arriaza J, et al. PLoS One. 2013 Jun 27;8(6):e66894. doi: 10.1371/journal.pone.0066894. Print 2013. PLoS One. 2013. PMID: 23826170 Free PMC article. - Mutational analysis of vaccinia virus E3 protein: the biological functions do not correlate with its biochemical capacity to bind double-stranded RNA.
Dueck KJ, Hu YS, Chen P, Deschambault Y, Lee J, Varga J, Cao J. Dueck KJ, et al. J Virol. 2015 May;89(10):5382-94. doi: 10.1128/JVI.03288-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740987 Free PMC article. - Human Host Range Restriction of the Vaccinia Virus C7/K1 Double Deletion Mutant Is Mediated by an Atypical Mode of Translation Inhibition.
Sivan G, Glushakow-Smith SG, Katsafanas GC, Americo JL, Moss B. Sivan G, et al. J Virol. 2018 Nov 12;92(23):e01329-18. doi: 10.1128/JVI.01329-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209174 Free PMC article. - Rendezvous with Vaccinia Virus in the Post-smallpox Era: R&D Advances.
Wang Y. Wang Y. Viruses. 2023 Aug 15;15(8):1742. doi: 10.3390/v15081742. Viruses. 2023. PMID: 37632084 Free PMC article. Review. - The complete genome sequences of poxviruses isolated from a penguin and a pigeon in South Africa and comparison to other sequenced avipoxviruses.
Offerman K, Carulei O, van der Walt AP, Douglass N, Williamson AL. Offerman K, et al. BMC Genomics. 2014 Jun 12;15:463. doi: 10.1186/1471-2164-15-463. BMC Genomics. 2014. PMID: 24919868 Free PMC article.
References
- Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. - PubMed
- Bablanian, R., B. Baxt, J. A. Sonnabend, and M. Esteban. 1978. Studies on the mechanisms of vaccinia virus cytopathic effects. II. Early cell rounding is associated with virus polypeptide synthesis. J. Gen. Virol. 39:403-413. - PubMed
- Bablanian, R., G. Coppola, S. Scribani, and M. Esteban. 1981. Inhibition of protein synthesis by vaccinia virus. III. The effect of ultraviolet-irradiated virus on the inhibition of protein synthesis. Virology 112:1-12. - PubMed
- Bablanian, R., G. Coppola, S. Scribani, and M. Esteban. 1981. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology 112:13-24. - PubMed
- Bablanian, R., M. Esteban, B. Baxt, and J. A. Sonnabend. 1978. Studies on the mechanisms of vaccinia virus cytopathic effects. I. Inhibition of protein synthesis in infected cells is associated with virus-induced RNA synthesis. J. Gen. Virol. 39:391-402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources