The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain - PubMed (original) (raw)
The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain
Hang Shi et al. Nat Struct Mol Biol. 2006 Jun.
Abstract
The mammalian retromer complex consists of SNX1, SNX2, Vps26, Vps29 and Vps35, and retrieves lysosomal enzyme receptors from endosomes to the trans-Golgi network. The structure of human Vps26A at 2.1-A resolution reveals two curved beta-sandwich domains connected by a polar core and a flexible linker. Vps26 has an unpredicted structural relationship to arrestins. The Vps35-binding site on Vps26 maps to a mobile loop spanning residues 235-246, near the tip of the C-terminal domain. The loop is phylogenetically conserved and provides a mechanism for Vps26 integration into the complex that leaves the rest of the structure free for engagements with membranes and for conformational changes. Hydrophobic residues and a glycine in this loop are required for integration into the retromer complex and endosomal localization of human Vps26, and for the function of yeast Vps26 in carboxypeptidase Y sorting.
Figures
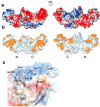
Figure 1. Structure of Vps26A
(a) Ribbon model of Vps26. Inner β-strands are colored in yellow and outer β-strands are colored in orange. The interdomain linker is colored in magenta. All structural figures were generated with Pymol (W. Delano,
). (b) Structure-based sequence alignment of Vps26 orthologs and arrestins (visual arrestin PDB: 1CF1 and β-arrestin 1 PDB: 1G4M). Residues involved in Vps35 binding are colored in red; polar core residues are labeled by open circles ○; hydrophobic residues in the interdomain contact are labeled by filled diamonds♦; acidic patch residues are labeled by daggers †; basic patch residues are labeled by filled circles•; yeast dominant negative mutants are labeled with an asterisk *; and the N-C domain linker is boxed in magenta.
Figure 2. Surface properties of Vps26
(a) Electrostatic potential. Surfaces are colored with saturating blue and red at ± 5kT/e. (b) Residues altered in site-directed mutagenesis (
Table 1) are colored in orange. (c) Potential membrane binding site showing in surface representation and colored with saturating blue and red at ± 5kT/e. Hydrophobic residues and positively charged residues are shown in orange balls and sticks.
Figure 3. Polar core of Vps26
(a) Polarity of the interface between two domains. Hydrophobic residues are colored in green, polar residues are colored in white. Acidic residues buried in the polar core are colored in red, uncharged residues involved in hydrogen bonding are colored magenta, and basic residues are colored in blue. (b) Electrostatic potential at the interface is colored with saturating blue and red at ± 5kT/e. (c) Residues contributing to the first cluster of polar interactions are shown in balls and sticks. Hydrogen bonds distances are in Å. N domain residues are colored in lime and C domain residues are colored in violet. (d) Residues contributing to the second cluster of polar interactions are shown in balls and sticks.
Figure 4. Structural similarities between Vps26 and β-arrestin
(a) Vps26 is shown in a ribbon model with the N domain colored in lime and the C domain in violet. The interdomain linker is colored blue. Polar core residues are colored in orange. (b) β -arrestin1 is shown in a ribbon model in the same color scheme as (a). The disordered region at the C tail is modeled with in an arbitrary conformation and colored in grey with the clathrin binding site shown in red spheres. The AP-2 binding site is shown in green spheres. The GPCR binding sites in the N and C domain cups are colored in purple. The PIP2 binding residues are shown in blue balls and sticks. The C domain lariat and the C terminal tail are colored in pink and yellow respectively. (c) Polar core residues in Vps26. (d) Polar core residues in β-arrestin1.
Figure 5. Identification of the Vps35 binding site on Vps26
(a) The interaction of Vps35 fused to Gal4BD with wild-type or mutant Vps26 constructs (numbered from 1 to 30 in Table 1) fused to Gal4AD was analyzed using the yeast two-hybrid system. The ability to grow in the absence of histidine (-His) is indicative of interactions. A summary of the results from three independent experiments is presented in Table 1 and a representative experiment is shown in the figure. Negative and positive controls for interactions described in Materials and Methods are also shown. Notice that the I235D M236D (#16) and Δ238–246 GG(#18)Vps26 transformants fail to grow on –His plates. (b) Whole cell extracts of the co-transformed cells from the yeast two-hybrid assay expressing Vps35 together with wild-type (WT) (lane 1), I235D M236D (#16, lane 2), Δ238–246 GG(#18)lane 3) and L250D F251D (#12, lane 4) Vps26 constructs, Vps35 and pGADT7 (lane 6), pGBKT7 and wild-type Vps26 (lane 5), pGBKT7 and pGADT7 (lane 7), or p53 and SV40 large T-antigen (Tag) (lane 8) were subjected to SDS-PAGE and immunoblotting using polyclonal antibodies to Vps35 (top panel) and Vps26 (bottom panel) (c) Additional Vps26 mutants were tested for interaction with Vps26 using the yeast two-hybrid system. A summary of the results is presented in Table 2 and a representative experiment is shown in the figure. Notice that the 238–246 polyS and G238P mutants of Vps26 fail to support growth on –His plates in the presence of the competitive inhibitor of the His3 protein, 3-aminotriazole (3-AT). (c) Residues involved in binding Vps26 are highlighted in a space-filling model. Residues that are disordered in the Vps26 structure were modeled in a sterically allowed but otherwise arbitrary conformation. In panel (a) BD: pGBKY7 and AD: pGADT7.
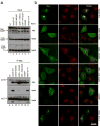
Figure 6. The binding site for Vps35 is required for Vps26 integration into the retromer complex in vivo
(a) Lysates from HeLa cells that were either not transfected (lanes 1 and 8, NT) or transfected with cDNAs encoding wild-type (lanes 2 and 9), IM-235,236-DD (lanes 3 and 10), Δ238–246 GG (lanes 4 and 11), 238–246 polyS (lanes 5 and 12), G238P (lanes 6 and 13) and R69A E71A (lanes 7 and 14) forms of myc-tagged Vps26 were subjected to immunoprecipitation (IP) using a mouse monoclonal antibody to the myc epitope The lysates (1% of the total, lanes 1–7) and immunoprecipitates (lanes 8–14) were subsequently analyzed by SDS-PAGE and immunoblotting (IB) with antibodies to the myc epitope (top panel), Vps35 (middle panel) or Vps29 (bottom panel). (b) The intracellular localization of Vps26-myc and the Vps26-myc mutants indicated in the figure (left column shows Alexa 488 green channel), as well as the colocalization of these constructs with SNX1 (middle column shows Alexa 546, red channel), was examined in fixed/permeabilized cells by indirect immunofluorescence and confocal microscopy. The right-hand column shows merged images; yellow indicates co-localization.
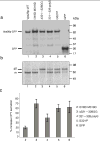
Figure 7. Analysis of CPY sorting in wild-type and mutant Vps26p-expressing yeast strains
(a) S. cerevisiae vps26Δ strains transformed with expression plasmids encoding wild-type (lane 1) or I318D M319D (lane 2), Δ321–328 GG lane3, 321–328 polyS (lane 4) or G321P (lane 5) Vps26-GFP constructs, or GFP (lane 6), were lysed and analyzed by SDS-PAGE and immunoblotting using anti-GFP antibody. (b) The processing of CPY in the same strains described in (a) was analyzed by Metabolic-labeling, pulse-chase analysis and immunoprecipitation with antibody to CPY. Lanes correspond to the same constructs mentioned in (a). The positions of the Golgi precursor (p2) and mature (m) forms of CPY are indicated (c) Secretion of CPY into the medium by S. cerevisiae vps26Δ strains transformed with expression plasmids encoding the mutant Vps26-GFP constructs I318D M319D, Δ321–328 GG, 321–318 polyS and G321P, or GFP, was analyzed using a colony blotting assay. Values represent the percent increase of CPY secretion relative to the CPY secreted by a wild-type-Vps26-GFP-transformed vps26Δ strain. Results are expressed as mean ± SEM from four independent experiments.
Similar articles
- Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors.
Rojas R, Kametaka S, Haft CR, Bonifacino JS. Rojas R, et al. Mol Cell Biol. 2007 Feb;27(3):1112-24. doi: 10.1128/MCB.00156-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101778 Free PMC article. - Structure of Vps26B and mapping of its interaction with the retromer protein complex.
Collins BM, Norwood SJ, Kerr MC, Mahony D, Seaman MN, Teasdale RD, Owen DJ. Collins BM, et al. Traffic. 2008 Mar;9(3):366-79. doi: 10.1111/j.1600-0854.2007.00688.x. Epub 2007 Dec 11. Traffic. 2008. PMID: 18088321 - Functional architecture of the retromer cargo-recognition complex.
Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH. Hierro A, et al. Nature. 2007 Oct 25;449(7165):1063-7. doi: 10.1038/nature06216. Epub 2007 Sep 23. Nature. 2007. PMID: 17891154 Free PMC article. - Retromer.
Bonifacino JS, Hurley JH. Bonifacino JS, et al. Curr Opin Cell Biol. 2008 Aug;20(4):427-36. doi: 10.1016/j.ceb.2008.03.009. Epub 2008 May 9. Curr Opin Cell Biol. 2008. PMID: 18472259 Free PMC article. Review. - Retromer: Structure, function, and roles in mammalian disease.
Trousdale C, Kim K. Trousdale C, et al. Eur J Cell Biol. 2015 Nov;94(11):513-21. doi: 10.1016/j.ejcb.2015.07.002. Epub 2015 Jul 17. Eur J Cell Biol. 2015. PMID: 26220253 Review.
Cited by
- Ubiquitin-mediated regulation of endocytosis by proteins of the arrestin family.
Becuwe M, Herrador A, Haguenauer-Tsapis R, Vincent O, Léon S. Becuwe M, et al. Biochem Res Int. 2012;2012:242764. doi: 10.1155/2012/242764. Epub 2012 Sep 4. Biochem Res Int. 2012. PMID: 22988512 Free PMC article. - Structure of a myosin•adaptor complex and pairing by cargo.
Shi H, Singh N, Esselborn F, Blobel G. Shi H, et al. Proc Natl Acad Sci U S A. 2014 Mar 25;111(12):E1082-90. doi: 10.1073/pnas.1401428111. Epub 2014 Feb 12. Proc Natl Acad Sci U S A. 2014. PMID: 24522109 Free PMC article. - Casein kinase 1 controls the activation threshold of an α-arrestin by multisite phosphorylation of the interdomain hinge.
Herrador A, Livas D, Soletto L, Becuwe M, Léon S, Vincent O. Herrador A, et al. Mol Biol Cell. 2015 Jun 1;26(11):2128-38. doi: 10.1091/mbc.E14-11-1552. Epub 2015 Apr 7. Mol Biol Cell. 2015. PMID: 25851600 Free PMC article. - A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport.
Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ. Steinberg F, et al. Nat Cell Biol. 2013 May;15(5):461-71. doi: 10.1038/ncb2721. Epub 2013 Apr 7. Nat Cell Biol. 2013. PMID: 23563491 Free PMC article. - Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis.
Zelazny E, Santambrogio M, Pourcher M, Chambrier P, Berne-Dedieu A, Fobis-Loisy I, Miège C, Jaillais Y, Gaude T. Zelazny E, et al. J Biol Chem. 2013 Mar 29;288(13):8815-25. doi: 10.1074/jbc.M112.440503. Epub 2013 Jan 29. J Biol Chem. 2013. PMID: 23362252 Free PMC article.
References
- Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: New twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. - PubMed
- Bonifacino JS. The GGA proteins: adaptors on the move. Nat Rev Mol Cell Biol. 2004;5:23–32. - PubMed
- Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. - PubMed
- Diaz E, Pfeffer SR. TIP47: A cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous