Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression - PubMed (original) (raw)
Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression
Eberhard Weihe et al. Cell Mol Neurobiol. 2006 Jul-Aug.
Abstract
1. We investigate here for the first time in primate brain the combinatorial expression of the three major functionally relevant proteins for catecholaminergic neurotransmission tyrosine hydroxylase (TH), aromatic acid acid decarboxylase (AADC), and the brain-specific isoform of the vesicular monoamine transporter, VMAT2, using highly specific antibodies and immunofluorescence with confocal microscopy to visualize combinatorial expression of these proteins. 2. In addition to classical TH, AADC, and VMAT2-copositive catecholaminergic neurons, two unique kinds of TH-positive neurons were identified based on co-expression of AADC and VMAT2. 3. TH and AADC co-positive, but VMAT2-negative neurons, are termed "nonexocytotic catecholaminergic TH neurons." These were found in striatum, olfactory bulb, cerebral cortex, area postrema, nucleus tractus solitarius, and in the dorsal motor nucleus of the vagus. 4. TH-positive neurons expressing neither AADC nor VMAT2 are termed "dopaergic TH neurons." We identified these neurons in supraoptic, paraventricular and periventricular hypothalamic nuclei, thalamic paraventicular nucleus, habenula, parabrachial nucleus, cerebral cortex and spinal cord. We were unable to identify any dopaergic (TH-positive, AADC-negative) neurons that expressed VMAT2, suggesting that regulatory mechanisms exist for shutting off VMAT2 expression in neurons that fail to biosynthesize its substrates. 5. In several cases, the corresponding TH phenotypes were identified in the adult rat, suggesting that this rodent is an appropriate experimental model for further investigation of these TH-positive neuronal cell groups in the adult central nervous system. Thus, no examples of TH and VMAT2 co-positive neurons lacking AADC expression were found in rodent adult nervous system. 6. In conclusion, the adult mammalian nervous system contains in addition to classical catecholaminergic neurons, cells that can synthesize dopamine, but cannot transport and store it in synaptic vesicles, and neurons that can synthesize only L-dopa and lack VMAT2 expression. The presence of these additional populations of TH-positive neurons in the adult primate CNS has implications for functional catecholamine neurotransmission, its derangement in disease and drug abuse, and its rescue by gene therapeutic maneuvers in neurodegenerative diseases such as Parkinson's disease.
Figures
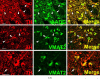
Fig. 1
Coexpression of VMAT2 and catecholamine synthesizing enzymes in classical catecholaminergic cell groups of rhesus monkey brain. Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. (A) Dopaminergic TH-positive neurons and processes of the substantia nigra pars compacta co-stain for AADC and VMAT2 (arrow heads). Note that VMAT2 staining in neuronal processes and fibers is comparatively weak and absent from some TH-positive fiber profiles. VMAT2-immunoreactivity appears granular and is asymmetrically concentrated in the perinuclear space while TH-positive staining comprises the entire cell bodies (lower panel). Note VMAT2-positive varicose fiber profiles lacking TH (arrows in lower panel). Bars are 30 _μ_m. (B) Noradrenergic TH-positive neurons and processes of the locus coeruleus co-stain for AADC, DBH, and VMAT2 (arrow heads). Note two small AADC-positive cell profiles that lack TH (arrows). VMAT2-positive/TH-negative puncta are intermingled between TH-positive/VMAT2-positive neuronal cell bodies and processes (high magnification in lower panel). The VMAT2-positive/TH-negative puncta presumably represent serotonergic input. The upper three panels are adjacent sections showing the same blood vessel (asterisks). Note staining of portions or all of the region of the nucleus in some AADC-positive cells, which can be attributed to superimposition of positive staining in cytoplasm underlying or overlaying the nucleus in these 7–10 _μ_m sections. Bars are 30 _μ_m.
Fig. 1
Coexpression of VMAT2 and catecholamine synthesizing enzymes in classical catecholaminergic cell groups of rhesus monkey brain. Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. (A) Dopaminergic TH-positive neurons and processes of the substantia nigra pars compacta co-stain for AADC and VMAT2 (arrow heads). Note that VMAT2 staining in neuronal processes and fibers is comparatively weak and absent from some TH-positive fiber profiles. VMAT2-immunoreactivity appears granular and is asymmetrically concentrated in the perinuclear space while TH-positive staining comprises the entire cell bodies (lower panel). Note VMAT2-positive varicose fiber profiles lacking TH (arrows in lower panel). Bars are 30 _μ_m. (B) Noradrenergic TH-positive neurons and processes of the locus coeruleus co-stain for AADC, DBH, and VMAT2 (arrow heads). Note two small AADC-positive cell profiles that lack TH (arrows). VMAT2-positive/TH-negative puncta are intermingled between TH-positive/VMAT2-positive neuronal cell bodies and processes (high magnification in lower panel). The VMAT2-positive/TH-negative puncta presumably represent serotonergic input. The upper three panels are adjacent sections showing the same blood vessel (asterisks). Note staining of portions or all of the region of the nucleus in some AADC-positive cells, which can be attributed to superimposition of positive staining in cytoplasm underlying or overlaying the nucleus in these 7–10 _μ_m sections. Bars are 30 _μ_m.
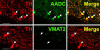
Fig. 2
Absence of VMAT2 expression from dopaminergic TH-positive neurons in rhesus monkey ol-factory bulb. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. TH-positive neuronal and cell fiber profiles co-express AADC, but lack VMAT2 (arrows). Note VMAT2-positive/TH-negative puncta which presumably represent serotonergic input. Bars are 30 _μ_m.
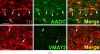
Fig. 3
Heterogeneous phenotypes of presumed dopaminergic neurons in rhesus monkey area postrema (AP) and nucleus of the solitary tract (NTS). Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. (A) The majority of TH-positive neurons of the AP coexpress AADC (double arrow heads in upper panel), but none of them coexpresses VMAT2 (arrows in lower panel). Note occurrence of both AADC-positive/TH-negative (arrow head) and TH-positive/AADC-negative (arrow) neurons (upper panel). VMAT2-positive/TH-positive and VMAT2-positive/TH-negative puncta are encountered. Bars are 30 _μ_m. (B) TH-positive neurons in the NTS coexpress AADC (arrows in upper panel), but lack VMAT2 (arrows in lower panel). In addition, AADC-positive/TH-negative neurons (arrow heads in upper panel) and VMAT2-positive/TH-negative neurons and fibers (arrow heads in lower panel) are encountered. Upper and lower panels are from adjacent sections. Asterisks label the same blood vessel profile. As in Fig. 1, staining in the vicinity of the nucleus in some cells is attributable to overlaying or underlying positively stained cytoplasm for the soluble enzyme AADC. Bars are 100 _μ_m.
Fig. 3
Heterogeneous phenotypes of presumed dopaminergic neurons in rhesus monkey area postrema (AP) and nucleus of the solitary tract (NTS). Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. (A) The majority of TH-positive neurons of the AP coexpress AADC (double arrow heads in upper panel), but none of them coexpresses VMAT2 (arrows in lower panel). Note occurrence of both AADC-positive/TH-negative (arrow head) and TH-positive/AADC-negative (arrow) neurons (upper panel). VMAT2-positive/TH-positive and VMAT2-positive/TH-negative puncta are encountered. Bars are 30 _μ_m. (B) TH-positive neurons in the NTS coexpress AADC (arrows in upper panel), but lack VMAT2 (arrows in lower panel). In addition, AADC-positive/TH-negative neurons (arrow heads in upper panel) and VMAT2-positive/TH-negative neurons and fibers (arrow heads in lower panel) are encountered. Upper and lower panels are from adjacent sections. Asterisks label the same blood vessel profile. As in Fig. 1, staining in the vicinity of the nucleus in some cells is attributable to overlaying or underlying positively stained cytoplasm for the soluble enzyme AADC. Bars are 100 _μ_m.
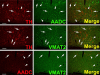
Fig. 4
Absence of VMAT2 from intrinsic TH-positive dopaminergic neurons of rhesus monkey striatum. Each horizontal panel (row) represents a single co-stained section analyzed by confocal microscopy. Adjacent sections show a TH-positive neuron costaining for AADC (upper panel), but lacking VMAT2 (lower panel) (arrows). Note that the vast majority of TH-positive dopaminergic input from the substantia nigra co-stains for AADC and VMAT2 (arrow heads). The AADC-positive/TH-negative and VMAT2-positive/TH-negative fibers/puncta are presumed to represent serotonergic input to the striatum (double arrow heads). Bars are 30 _μ_m.
Fig. 5
DOPAergic neurons in rhesus monkey lateral parabrachial nucleus and spinal cord. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. (A) TH-positive neurons in the lateral parabrachial nucleus are negative for AADC and VMAT2 (arrows). In addition to some TH-positive/AADC-positive and TH-positive/VMAT2-positive varicose fibers and puncta, AADC-positive/TH-negative and VMAT2-positive/TH-negative fibers and puncta are present (presumed serotonergic input). Asterisks label profiles of the same blood vessel. Bars are 30 _μ_m. (B) TH-positive neurons in the intermediolateral cell column of the thoracic spinal cord lack AADC and VMAT2 (arrows). AADC-positive varicose fibers containing TH (double arrow heads in middle panel) and lacking TH (arrow heads in middle panel) are present. VMAT2-positive varicose fibers/puncta containing TH (double arrow heads in lower panel) and lacking TH (arrow heads in lower panel) are encountered. Note also TH-positive fibers lacking VMAT2 (uppermost arrow in lower panel). Bars are 30 _μ_m.
Fig. 5
DOPAergic neurons in rhesus monkey lateral parabrachial nucleus and spinal cord. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. (A) TH-positive neurons in the lateral parabrachial nucleus are negative for AADC and VMAT2 (arrows). In addition to some TH-positive/AADC-positive and TH-positive/VMAT2-positive varicose fibers and puncta, AADC-positive/TH-negative and VMAT2-positive/TH-negative fibers and puncta are present (presumed serotonergic input). Asterisks label profiles of the same blood vessel. Bars are 30 _μ_m. (B) TH-positive neurons in the intermediolateral cell column of the thoracic spinal cord lack AADC and VMAT2 (arrows). AADC-positive varicose fibers containing TH (double arrow heads in middle panel) and lacking TH (arrow heads in middle panel) are present. VMAT2-positive varicose fibers/puncta containing TH (double arrow heads in lower panel) and lacking TH (arrow heads in lower panel) are encountered. Note also TH-positive fibers lacking VMAT2 (uppermost arrow in lower panel). Bars are 30 _μ_m.
Fig. 6
DOPAergic neurons in the rat habenular nucleus. Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. TH-positive neurons lack AADC (arrows in upper panel) and VMAT2 (arrows in lower panel). Some TH-positive varicose fibers costain for AADC (double arrow heads in upper panel). Presumed serotonergic AADC-positive fibers lack TH (arrow head in upper panel). Presumed serotonergic VMAT2-positive fibers lack TH (arrow head in lower panel). Bars are 30 _μ_m.
Fig. 7
Absence of AADC and VMAT2 from TH-positive neurons in rat supraoptic nucleus (SON). Horizontal panels (rows) represent single co-stained sections adjacent to each other analyzed by confocal microscopy. TH-positive neurons in the SON lack AADC (arrows in upper panel) and VMAT2 (arrows in middle panel). AADC-positive neurons dorsal to the SON and fibers in the SON are TH-negative (arrow heads in upper panel). AADC-positive neurons dorsal to the SON also lack VMAT2 (arrows in lower panel). Note VMAT2-positive/TH-positive fibers (double arrow heads in middle panel) and VMAT2-positive/TH-negative fibers (arrow heads in middle panel) in the SON. Some VMAT2-positive fibers in and at the dorsal border of the SON are AADC- (arrow heads in lower panel) and TH-negative (arrow heads in middle panel) and are presumed to represent histaminergic input to the SON region. Bars are 100 _μ_m.
Fig. 8
Heterogeneous phenotypes of TH + neurons in the rhesus monkey periventricular hypothalamus. Horizontal panels (rows) represent single co-stained sections analyzed by confocal microscopy. Upper panel: TH-positive neurons coexpressing AADC (double arrow heads) and TH-positive/AADC-negative neurons (arrows) are present. Note AADC-positive/TH-negative fiber (arrow head). Lower panel: TH-positive neurons lack VMAT2 (arrows). Note VMAT2-positive/TH-positive and VMAT2-positive/TH-negative puncta. Occasional immunopositivity in the region of the nucleus for the cytoplasmic antigen AADC is attributed to superimposition of cytoplasm above or below the nucleus in these sections. Bars are 30 _μ_m.
Fig. 9
Heterogeneous phenotypes of TH-positive neurons in the rhesus monkey paraventricular thalamic nucleus. Horizontal panels (rows) represent single co-stained section adjacent to each other analyzed by confocal microscopy. AADC and VMAT2 are absent from TH-positive neurons (arrows in upper and lower panels, respectively). Note AADC-positive/TH-negative fibers (arrow heads in upper panel) and VMAT2-positive/TH-negative fibers (arrow heads in lower panel) as well as VMAT2-positive/TH-positive fibers (double arrow heads in lower panel). Asterisks label third ventricle. Bars are 50 _μ_m.
Similar articles
- Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties.
Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Li X, et al. Brain Struct Funct. 2013 Sep;218(5):1159-76. doi: 10.1007/s00429-012-0452-z. Epub 2012 Aug 29. Brain Struct Funct. 2013. PMID: 22926514 - Neurons expressing individual enzymes of dopamine synthesis in the mediobasal hypothalamus of adult rats: functional significance and topographic interrelations.
Ugrumov M, Taxi J, Pronina T, Kurina A, Sorokin A, Sapronova A, Calas A. Ugrumov M, et al. Neuroscience. 2014 Sep 26;277:45-54. doi: 10.1016/j.neuroscience.2014.06.051. Epub 2014 Jul 2. Neuroscience. 2014. PMID: 24997271 - Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance.
Ugrumov MV. Ugrumov MV. J Chem Neuroanat. 2009 Dec;38(4):241-56. doi: 10.1016/j.jchemneu.2009.08.004. Epub 2009 Aug 19. J Chem Neuroanat. 2009. PMID: 19698780 Review. - L-amino acid decarboxylase- and tyrosine hydroxylase-immunoreactive cells in the extended olfactory amygdala and elsewhere in the adult prairie vole brain.
Ahmed EI, Northcutt KV, Lonstein JS. Ahmed EI, et al. J Chem Neuroanat. 2012 Jan;43(1):76-85. doi: 10.1016/j.jchemneu.2011.10.006. Epub 2011 Nov 2. J Chem Neuroanat. 2012. PMID: 22074805 - Brain neurons partly expressing dopaminergic phenotype: location, development, functional significance, and regulation.
Ugrumov MV. Ugrumov MV. Adv Pharmacol. 2013;68:37-91. doi: 10.1016/B978-0-12-411512-5.00004-X. Adv Pharmacol. 2013. PMID: 24054140 Review.
Cited by
- Dopamine in Auditory Nuclei and Lemniscal Projections is Poised to Influence Acoustic Integration in the Inferior Colliculus.
Harris S, Afram R, Shimano T, Fyk-Kolodziej B, Walker PD, Braun RD, Holt AG. Harris S, et al. Front Neural Circuits. 2021 Mar 5;15:624563. doi: 10.3389/fncir.2021.624563. eCollection 2021. Front Neural Circuits. 2021. PMID: 33746717 Free PMC article. - Gestational diabetes mellitus increased the number of dopaminergic neurons in the olfactory bulb of rat offspring.
Nazari Z, Bahrehbar K, Ghafari S, Golalipour MJ. Nazari Z, et al. Iran J Basic Med Sci. 2023;26(12):484-1489. doi: 10.22038/IJBMS.2023.71300.15488. Iran J Basic Med Sci. 2023. PMID: 37970447 Free PMC article. - A region-dependent allele-biased expression of Dopa decarboxylase in mouse brain.
Sheng KY, Nakano T, Yamaguchi S. Sheng KY, et al. Front Cell Dev Biol. 2022 Dec 7;10:1078927. doi: 10.3389/fcell.2022.1078927. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36568970 Free PMC article. - Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson's disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS).
Matusch A, Depboylu C, Palm C, Wu B, Höglinger GU, Schäfer MK, Becker JS. Matusch A, et al. J Am Soc Mass Spectrom. 2010 Jan;21(1):161-71. doi: 10.1016/j.jasms.2009.09.022. Epub 2009 Oct 6. J Am Soc Mass Spectrom. 2010. PMID: 19892565 - Nanostructure lipid carriers enhance alpha-mangostin neuroprotective efficacy in mice with rotenone-induced neurodegeneration.
Sakamula R, Yata T, Thong-Asa W. Sakamula R, et al. Metab Brain Dis. 2022 Jun;37(5):1465-1476. doi: 10.1007/s11011-022-00967-w. Epub 2022 Mar 30. Metab Brain Dis. 2022. PMID: 35353275
References
- Andresen MC, Doyle MW, Jin YH, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann. N.Y. Acad. Sci. 2001;940:132–141. - PubMed
- Anlauf M, Schäfer MK-H, Eiden LE, Weihe E. Chemical coding of the human gastrointestinal nervous system: Cholinergic, VIPergic and catecholaminergic phenotypes. J. Comp. Neurol. 2003;459:90–111. - PubMed
- Axelrod J. Doing research in the Intramural Program of the National Institutes of Health. Perspect. Biol. Med. 1986;29:S131–S137. - PubMed
- Balan IS, Ugrumov MV, Calas A, Mailly P, Kreiger M, Thibault J. Tyrosine hydroxylase-expressing and/or aromatic L-amino acid decarboxylase-expressing neurons in the mediobasal hypothalamus of perinatal rats: differentiation and sexual dimorphism. J. Comp. Neurol. 2000;425:167–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources