Structure of trichamide, a cyclic peptide from the bloom-forming cyanobacterium Trichodesmium erythraeum, predicted from the genome sequence - PubMed (original) (raw)
Structure of trichamide, a cyclic peptide from the bloom-forming cyanobacterium Trichodesmium erythraeum, predicted from the genome sequence
Sebastian Sudek et al. Appl Environ Microbiol. 2006 Jun.
Abstract
A gene cluster for the biosynthesis of a new small cyclic peptide, dubbed trichamide, was discovered in the genome of the global, bloom-forming marine cyanobacterium Trichodesmium erythraeum ISM101 because of striking similarities to the previously characterized patellamide biosynthesis cluster. The tri cluster consists of a precursor peptide gene containing the amino acid sequence for mature trichamide, a putative heterocyclization gene, an oxidase, two proteases, and hypothetical genes. Based upon detailed sequence analysis, a structure was predicted for trichamide and confirmed by Fourier transform mass spectrometry. Trichamide consists of 11 amino acids, including two cysteine-derived thiazole groups, and is cyclized by an N C terminal amide bond. As the first natural product reported from T. erythraeum, trichamide shows the power of genome mining in the prediction and discovery of new natural products.
Figures
FIG. 1.
The tri gene cluster. Arrows denote ORFs and their direction. Black ORFs are tRNA synthetases, white ORFs are conserved hypothetical genes without a homolog in the pat cluster, green ORFs are pat homologs, and the gene encoding the precursor peptide is orange.
FIG. 2.
Alignment of the precursor peptides PatE and TriG. The sequences encoding patellamide C, patellamide A, and trichamide (top to bottom) are underlined, and the proposed cyclization signals are in boldface type.
FIG. 3.
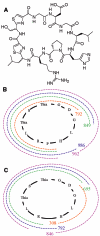
(A) Structure of trichamide. Stereochemistry is inferred and not determined experimentally, as described in the text. (B) Assignment of CID-MS fragments from Table 2 to the trichamide structure. (C) Assignment of IRMPD-MS fragments.
FIG. 4.
Proposed biosynthetic pathway to trichamide.
Similar articles
- Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella.
Schmidt EW, Nelson JT, Rasko DA, Sudek S, Eisen JA, Haygood MG, Ravel J. Schmidt EW, et al. Proc Natl Acad Sci U S A. 2005 May 17;102(20):7315-20. doi: 10.1073/pnas.0501424102. Epub 2005 May 9. Proc Natl Acad Sci U S A. 2005. PMID: 15883371 Free PMC article. - Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria.
Sivonen K, Leikoski N, Fewer DP, Jokela J. Sivonen K, et al. Appl Microbiol Biotechnol. 2010 May;86(5):1213-25. doi: 10.1007/s00253-010-2482-x. Epub 2010 Feb 27. Appl Microbiol Biotechnol. 2010. PMID: 20195859 Free PMC article. Review. - Overexpression and characterization of an iron storage and DNA-binding Dps protein from Trichodesmium erythraeum.
Castruita M, Saito M, Schottel PC, Elmegreen LA, Myneni S, Stiefel EI, Morel FM. Castruita M, et al. Appl Environ Microbiol. 2006 Apr;72(4):2918-24. doi: 10.1128/AEM.72.4.2918-2924.2006. Appl Environ Microbiol. 2006. PMID: 16597998 Free PMC article. - Hassallidins, antifungal glycolipopeptides, are widespread among cyanobacteria and are the end-product of a nonribosomal pathway.
Vestola J, Shishido TK, Jokela J, Fewer DP, Aitio O, Permi P, Wahlsten M, Wang H, Rouhiainen L, Sivonen K. Vestola J, et al. Proc Natl Acad Sci U S A. 2014 May 6;111(18):E1909-17. doi: 10.1073/pnas.1320913111. Epub 2014 Apr 17. Proc Natl Acad Sci U S A. 2014. PMID: 24742428 Free PMC article. - Circular micro-proteins and mechanisms of cyclization.
Conlan BF, Anderson MA. Conlan BF, et al. Curr Pharm Des. 2011 Dec;17(38):4318-28. doi: 10.2174/138161211798999410. Curr Pharm Des. 2011. PMID: 22204430 Review.
Cited by
- Spatial and Temporal Resolution of Cyanobacterial Bloom Chemistry Reveals an Open-Ocean Trichodesmium thiebautii as a Talented Producer of Specialized Metabolites.
Via CW, Grauso L, McManus KM, Kirk RD, Kim AM, Webb EA, Held NA, Saito MA, Scarpato S, Zimba PV, Moeller PDR, Mangoni A, Bertin MJ. Via CW, et al. Environ Sci Technol. 2024 Jun 4;58(22):9525-9535. doi: 10.1021/acs.est.3c10739. Epub 2024 May 17. Environ Sci Technol. 2024. PMID: 38758591 Free PMC article. - The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987-2020).
Hai Y, Wei MY, Wang CY, Gu YC, Shao CL. Hai Y, et al. Mar Life Sci Technol. 2021 May 19;3(4):488-518. doi: 10.1007/s42995-021-00101-2. eCollection 2021 Nov. Mar Life Sci Technol. 2021. PMID: 37073258 Free PMC article. Review. - Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties.
Nandagopal P, Steven AN, Chan LW, Rahmat Z, Jamaluddin H, Mohd Noh NI. Nandagopal P, et al. Biology (Basel). 2021 Oct 18;10(10):1061. doi: 10.3390/biology10101061. Biology (Basel). 2021. PMID: 34681158 Free PMC article. Review. - Phototrophic Co-cultures From Extreme Environments: Community Structure and Potential Value for Fundamental and Applied Research.
Shaw C, Brooke C, Hawley E, Connolly MP, Garcia JA, Harmon-Smith M, Shapiro N, Barton M, Tringe SG, Glavina Del Rio T, Culley DE, Castenholz R, Hess M. Shaw C, et al. Front Microbiol. 2020 Nov 6;11:572131. doi: 10.3389/fmicb.2020.572131. eCollection 2020. Front Microbiol. 2020. PMID: 33240229 Free PMC article. - Challenges and advances in genome mining of ribosomally synthesized and post-translationally modified peptides (RiPPs).
Zhong Z, He B, Li J, Li YX. Zhong Z, et al. Synth Syst Biotechnol. 2020 Jun 24;5(3):155-172. doi: 10.1016/j.synbio.2020.06.002. eCollection 2020 Sep. Synth Syst Biotechnol. 2020. PMID: 32637669 Free PMC article. Review.
References
- Capone, D. G., J. P. Zehr, H. W. Paerl, B. Bergmann, and E. J. Carpenter. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science 276:1221-1229.
- Carmichael, W. W. 1992. Cyanobacteria secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 72:445-459. - PubMed
- Devassy, V. P., P. M. Bhattathiri, and S. Z. Qasim. 1979. Succession of organisms following Trichodesmium phenomenon. Indian J. Mar. Sci. 8:88-93.
- Fu, X., T. Do, F. J. Schmitz, V. Andrusevich, and M. H. Engel. 1998. New cyclic peptides from the ascidian Lissoclinum patella. J. Nat. Prod. 61:1547-1551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials