SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation - PubMed (original) (raw)
SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation
Jennifer L Cannons et al. J Exp Med. 2006.
Abstract
X-linked lymphoproliferative disease is caused by mutations affecting SH2D1A/SAP, an adaptor that recruits Fyn to signal lymphocyte activation molecule (SLAM)-related receptors. After infection, SLAM-associated protein (SAP)-/- mice show increased T cell activation and impaired humoral responses. Although SAP-/- mice can respond to T-independent immunization, we find impaired primary and secondary T-dependent responses, with defective B cell proliferation, germinal center formation, and antibody production. Nonetheless, transfer of wild-type but not SAP-deficient CD4 cells rescued humoral responses in reconstituted recombination activating gene 2-/- and SAP-/- mice. To investigate these T cell defects, we examined CD4 cell function in vitro and in vivo. Although SAP-deficient CD4 cells have impaired T cell receptor-mediated T helper (Th)2 cytokine production in vitro, we demonstrate that the humoral defects can be uncoupled from cytokine expression defects in vivo. Instead, SAP-deficient T cells exhibit decreased and delayed inducible costimulator (ICOS) induction and heightened CD40L expression. Notably, in contrast to Th2 cytokine defects, humoral responses, ICOS expression, and CD40L down-regulation were rescued by retroviral reconstitution with SAP-R78A, a SAP mutant that impairs Fyn binding. We further demonstrate a role for SLAM/SAP signaling in the regulation of early surface CD40L expression. Thus, SAP affects expression of key molecules required for T-B cell collaboration by mechanisms that are distinct from its role in cytokine regulation.
Figures
Figure 1.
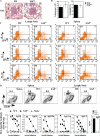
SAP−/− mice exhibit impaired immune responses to T-dependent antigens. (A) Production of NP-specific antibodies 21 d after NP-LPS (T-independent) immunization by ELISA. (B) Mice were immunized with a T-dependent antigen (NP-KLH) and challenged 70 d after primary immunization (n = 4–5 mice/time point/group). (C) GC development evaluated by gating on B220+IgDlo cells: GC B cells are FashiGL-7hi. (D) NP-specific [NP-(30)] antibody detected by ELISA. Ribi refers to adjuvant control. Day 0 of secondary response is 70 d after primary immunization. Note that y-axis NP-specific antibody dilution refers to the midpoint of the dilution curve for each genotype. Axes for each isotype are different. (E) Mice were immunized with SRBCs (Fig. S1) and injected with BrdU 5 h before they were killed. The numbers of dividing splenic CD19+ B cells and CD4+ T cells were determined by staining with anti-BrdU antibody.
Figure 2.
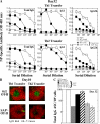
WT CD4 cells rescued antibody production in RAG2−/− recipients. RAG2−/− reconstitution experiments: SAP-deficient or WT CD4 T cells and either WT B cells (left panels A, C, E, and F) or SAP-deficient B cells (right panels B, D, G, and H) were cotransferred into RAG2−/− hosts (n = 4 mice/time point/group). Note that transfer experiments with WT or SAP-deficient B cells are separate experiments (see Fig. S3 for direct comparison). (A and B) GC development assessed by gating on B220+IgDlo cells and staining for FashiGL-7hi cells. (C and D) SRBC-specific antibody response evaluated by ELISA. Nonimmunized control refers to mice that received T and B lymphocytes but were not immunized. (E and G) Mice were injected with BrdU 5 h before they were killed to assess CD19+ B cell and CD4+ T cell proliferation. (F and H) Total bone marrow IgG+ plasma cells (ASC) on day 25 were quantified by ELISPOT.
Figure 3.
Impaired humoral immunity in response to S. mansoni egg injection. (A) Giemsa-stained lung section: granuloma formation 8 d after secondary injection. (B) Granuloma volume and percent eosinophil content. (C) Pooled mediastinal lymph nodes or splenocytes were cultured with 20 μg SEA for 72 h. For intracellular cytokine analysis, cells were rested for 18 h and restimulated with 3 ng/ml PMA and 1 μg/ml ionomycin. (D) Lymph nodes and splenocytes were stained for GC markers by gating on B220+IgDlo cells: GC B cells are FashiCD38int. (E) SEA-specific antibodies evaluated 8 d after challenge with S. mansoni eggs by ELISA. Note the different scales of the axes. *, antibody levels not detected. Data represent one of two independent experiments (n = 5 mice/group).
Figure 4.
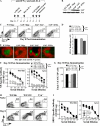
SAP-deficient Th2-differentiated antigen-specific CD4 T cells fail to rescue humoral defects. Th1- or Th2-skewed WT OT-II and SAP−/− OT-II CD4 cells were adoptively transferred to SAP−/− hosts that were subsequently immunized with NP-OVA. WT and SAP−/− controls did not receive transferred cells but were immunized. Data represent one of three independent experiments (n = 3–4 mice/time point/group). (A) [NP-(30)]-specific antibodies detected 32 d after immunization by ELISA. (B) GCs were detected in SAP−/− hosts reconstituted with WT but not SAP-deficient OT-II cells via staining with IgD (red) and GL-7 (green) 10 d after immunization. (C) Total bone marrow IgG+ plasma cells (ASC) were quantified by ELISPOT.
Figure 5.
Heightened and sustained CD40L surface expression on SAP-deficient CD4 cells. (A–C) WT and SAP−/− AND CD4 cells were stimulated with peptide-pulsed (1,000 nM PCC) P13.9 cells and evaluated for activation markers. (A) SAP−/− AND cell display enhanced and sustained levels of CD40L. (B) Percentage of Vα11+CD4+ cells stained for CD40L (left) and SLAM (right) expression. Mean fluorescence intensity for CD40L and SLAM are shown in Fig. S4. (C and D) SAP-deficient cells display elevated CD40L mRNA. RNA was isolated from WT and SAP-deficient AND CD4 cells after stimulation with peptide-pulsed P13.9 APCs and subjected to (C) real-time quantitative PCR and (D) RT-PCR analysis. (E) SLAM expression on P13.9 APCs reduced expression of CD40L on WT AND cells. (F) SLAM expression on P13.9 APCs did not affect mRNA levels after stimulation as assessed by RT-PCR.
Figure 6.
Impaired ICOS expression on SAP-deficient CD4 cells in vitro and in vivo. (A–C) WT and SAP−/−AND CD4 cells were stimulated with peptide-pulsed (1,000 nM PCC) P13.9 cells and evaluated for ICOS expression. (A) Expression and kinetics of ICOS measured by flow cytometry. (B) Percentage of Vα11+CD4+ cells stained positive for ICOS expression and mean fluorescence intensity of ICOS. (C) CFSE-labeled WT OT-II or SAP−/− OT-II T cells were adoptively transferred to SAP−/− hosts that were subsequently immunized with either PBS control or NP-OVA. Data represent one of three independent experiments (n = 2 mice/group). One representative example is shown for each genotype. Labeled cells were evaluated for expression of SLAM and ICOS 2 d after immunization.
Figure 7.
GC formation and antibody responses in SAP−/− mice are less dependent on SAP-mediated recruitment of Fyn. (A) WT OT-II and SAP−/− OT-II CD4 cells were retrovirally reconstituted with Migr control, WT SAP, or SAP-R78A vectors and transferred to SAP−/− mice that were subsequently immunized with NP-OVA. Data represent three independent experiments (n = 3 mice/time point/group). (B) SAP expression was verified by immunoblot. (C) GC B cells were detected with the transfer of WT (Migr) and SAP−/− cells reconstituted with WT SAP or SAP-R78A, but not Migr control. GC development assessed at day 8 after immunization by gating on B220+IgDlo cells: GC B cells are FashiGL-7hi. (D) Quantification of PNA+ B cells based on flow cytometric analysis.(E) GCs were detected in SAP−/− hosts via staining with IgD (red) and GL-7 (green) 8 d after immunization. (F) [NP-(30)]-specific antibodies detected 30 d after immunization by ELISA. (G) Total bone marrow IgG+ plasma cells (ASC) were quantified by ELISPOT. (H and I) Fyn−/− mice exhibit a productive humoral response to a T-dependent antigen, SRBCs. Data represent two independent experiments (n = 4–5 mice/time point/group). (H) GC development evaluated by gating on B220+IgDlo cells and staining for FashiGL-7hi expression. (I) SRBC-specific antibody response evaluated by ELISA 30 d after immunization. Fyn−/− mice are on a mixed 129/Sv × C57BL/6 background. Controls from 129/Sv and C57BL/6 strains are shown.
Figure 8.
Reexpression of SAP or SAP-R78A rescued aberrant CD40L and ICOS expression. SAP-deficient AND CD4 cells were reconstituted with WT SAP, SAP-R78A, or the Migr control vector. WT AND CD4 cells were reconstituted with Migr. Cells were restimulated with 100 nM PCC-pulsed P13.9 cells. Data represent four independent experiments. (A) SAP protein expression confirmed by immunoblot. (B and C) Expression of either WT SAP or SAP-R78A reduced basal and activated CD40L mRNA expression. RNA was isolated after stimulation with peptide-pulsed P13.9 APCs and subject to (B) RT-PCR and (C) real-time quantitative PCR analysis. (D and E) SAP and SAP-R78A, but not Migr control, improved CD40L kinetics and ICOS up-regulation in reconstituted SAP-deficient cells. Flow cytometric analysis (gated on CD4+GFP+ cells) of CD40L (top), ICOS (middle), and OX40 (bottom) expression. (E) Overlaid data from the 24-h time point. Filled histograms represent unstimulated cells.
Similar articles
- SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase.
McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. McCausland MM, et al. J Immunol. 2007 Jan 15;178(2):817-28. doi: 10.4049/jimmunol.178.2.817. J Immunol. 2007. PMID: 17202343 - Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product.
Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Latour S, et al. Nat Immunol. 2001 Aug;2(8):681-90. doi: 10.1038/90615. Nat Immunol. 2001. PMID: 11477403 - Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice.
Graham DB, Bell MP, McCausland MM, Huntoon CJ, van Deursen J, Faubion WA, Crotty S, McKean DJ. Graham DB, et al. J Immunol. 2006 Jan 1;176(1):291-300. doi: 10.4049/jimmunol.176.1.291. J Immunol. 2006. PMID: 16365421 - Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules.
Ma CS, Nichols KE, Tangye SG. Ma CS, et al. Annu Rev Immunol. 2007;25:337-79. doi: 10.1146/annurev.immunol.25.022106.141651. Annu Rev Immunol. 2007. PMID: 17201683 Review. - The role of SAP and SLAM family molecules in the humoral immune response.
Ma CS, Deenick EK. Ma CS, et al. Ann N Y Acad Sci. 2011 Jan;1217:32-44. doi: 10.1111/j.1749-6632.2010.05824.x. Epub 2010 Nov 22. Ann N Y Acad Sci. 2011. PMID: 21091715 Review.
Cited by
- Antigen-specific responses and ANA production in B6.Sle1b mice: a role for SAP.
Jennings P, Chan A, Schwartzberg P, Wakeland EK, Yuan D. Jennings P, et al. J Autoimmun. 2008 Dec;31(4):345-53. doi: 10.1016/j.jaut.2008.08.002. Epub 2008 Oct 8. J Autoimmun. 2008. PMID: 18845419 Free PMC article. - SAP enables T cells to help B cells by a mechanism distinct from Th cell programming or CD40 ligand regulation.
Kamperschroer C, Roberts DM, Zhang Y, Weng NP, Swain SL. Kamperschroer C, et al. J Immunol. 2008 Sep 15;181(6):3994-4003. doi: 10.4049/jimmunol.181.6.3994. J Immunol. 2008. PMID: 18768854 Free PMC article. - Follicular helper T cells: lineage and location.
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Fazilleau N, et al. Immunity. 2009 Mar 20;30(3):324-35. doi: 10.1016/j.immuni.2009.03.003. Immunity. 2009. PMID: 19303387 Free PMC article. Review. - The peptide specificity of the endogenous T follicular helper cell repertoire generated after protein immunization.
Leddon SA, Sant AJ. Leddon SA, et al. PLoS One. 2012;7(10):e46952. doi: 10.1371/journal.pone.0046952. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077537 Free PMC article. - Negative Regulation of Humoral Immunity Due to Interplay between the SLAMF1, SLAMF5, and SLAMF6 Receptors.
Wang N, Halibozek PJ, Yigit B, Zhao H, O'Keeffe MS, Sage P, Sharpe A, Terhorst C. Wang N, et al. Front Immunol. 2015 Apr 14;6:158. doi: 10.3389/fimmu.2015.00158. eCollection 2015. Front Immunol. 2015. PMID: 25926831 Free PMC article.
References
- Nichols, K.E., C.S. Ma, J.L. Cannons, P.L. Schwartzberg, and S.G. Tangye. 2005. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol. Rev. 203:180–199. - PubMed
- Latour, S., G. Gish, C.D. Helgason, R.K. Humphreis, T. Pawson, and A. Veillette. 2001. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2:681–690. - PubMed
- Latour, S., R. Roncagalli, R. Chen, M. Bakinowski, X. Shi, P.L. Schwartzberg, D. Davidson, and A. Veillette. 2003. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat. Cell Biol. 5:149–154. - PubMed
- Chan, B., A. Lanyi, H.K. Song, J. Griesbach, M. Simarro-Grande, F. Poy, D. Howie, J. Sumegi, C. Terhorst, and M.J. Eck. 2003. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 5:155–160. - PubMed
- Sayos, J., C. Wu, M. Morra, N. Wang, X. Zhang, D. Allen, S. van Schaik, L. Notarangelo, R. Geha, M.G. Roncarolo, et al. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 395:462–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous