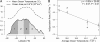Kinetic effects of temperature on rates of genetic divergence and speciation - PubMed (original) (raw)
Kinetic effects of temperature on rates of genetic divergence and speciation
Andrew P Allen et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Latitudinal gradients of biodiversity and macroevolutionary dynamics are prominent yet poorly understood. We derive a model that quantifies the role of kinetic energy in generating biodiversity. The model predicts that rates of genetic divergence and speciation are both governed by metabolic rate and therefore show the same exponential temperature dependence (activation energy of approximately 0.65 eV; 1 eV = 1.602 x 10(-19) J). Predictions are supported by global datasets from planktonic foraminifera for rates of DNA evolution and speciation spanning 30 million years. As predicted by the model, rates of speciation increase toward the tropics even after controlling for the greater ocean coverage at tropical latitudes. Our model and results indicate that individual metabolic rate is a primary determinant of evolutionary rates: approximately 10(13) J of energy flux per gram of tissue generates one substitution per nucleotide in the nuclear genome, and approximately 10(23) J of energy flux per population generates a new species of foraminifera.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
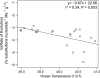
Fig. 1.
Effect of ocean temperature, 1/kT, on the size-corrected rate of neutral molecular evolution, ln(fo_α_M_1/4), for nuclear genomes of planktonic foraminifera. The slope (−0.67 eV) was fitted by using ordinary least-squares regression and is close to the value of −_E ≈ −0.65 eV (95% CI, −0.26 to −1.07 eV), which was predicted based on the temperature dependence of individual metabolic rate (Eq. 3). Refer to Appendix 1 for details on this global compilation of SSU rDNA data.
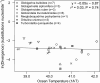
Fig. 2.
Effect of ocean temperature, 1/kT, on genetic divergence, ln(Ds), for nuclear genomes of ecologically distinct genotypes within seven morphospecies of planktonic foraminifera. The sample sizes in the legend refer to the numbers of pairwise comparisons among populations comprising each morphospecies. The data were weighted such that each morphospecies contributed equally to the ordinary least-squares regression slope, which does not differ from the predicted value of 0 (Eq. 7). This conclusion remains unchanged if data points, rather than morphospecies, are weighted equally (P = 0.10). Refer to Appendix 2 for details on this global compilation of SSU rDNA data.
Fig. 3.
Both ecological and macroevolutionary variables exhibit pronounced variation from the poles to the equator. (A) Depicted are the latitudinal gradient in contemporary mean annual sea-surface temperatures (48) (dashed line) and ocean surface area per 0.5° latitude (solid line; negative numbers correspond to southern latitudes). Different shades are used to represent four equal-area latitudinal bands of ≈9.1 × 107 km2 ocean area each. (B) Depicted are the effects of ocean temperature on time-averaged speciation rates over the past 30 Ma in each of the four equal-area latitudinal bands. The line was fitted by using ordinary least-squares regression. Speciation rates were calculated based on the latitudinal distribution of >150 FO of foraminifera morphospecies by using the Neptune database (32); 95% CIs (vertical lines) were generated, as described in Appendix 3, by using a randomization procedure that explicitly controls for the effects of variation in sampling efforts on paleontological analyses. The average sea-surface temperature within each latitudinal band over the past 30 Ma was estimated, as described in Appendix 4, by using a robust paleotemperature calibration (33).
Similar articles
- Speciation, Ecological Opportunity, and Latitude (American Society of Naturalists Address).
Schluter D. Schluter D. Am Nat. 2016 Jan;187(1):1-18. doi: 10.1086/684193. Epub 2015 Nov 19. Am Nat. 2016. PMID: 26814593 - Evolution of a Planktonic Foraminifer during Environmental Changes in the Tropical Oceans.
Ujiié Y, Ishitani Y. Ujiié Y, et al. PLoS One. 2016 Feb 17;11(2):e0148847. doi: 10.1371/journal.pone.0148847. eCollection 2016. PLoS One. 2016. PMID: 26886349 Free PMC article. - Population divergence in plant species reflects latitudinal biodiversity gradients.
Eo SH, Wares JP, Carroll JP. Eo SH, et al. Biol Lett. 2008 Aug 23;4(4):382-4. doi: 10.1098/rsbl.2008.0109. Biol Lett. 2008. PMID: 18492649 Free PMC article. - Environmental harshness is positively correlated with intraspecific divergence in mammals and birds.
Botero CA, Dor R, McCain CM, Safran RJ. Botero CA, et al. Mol Ecol. 2014 Feb;23(2):259-68. doi: 10.1111/mec.12572. Epub 2013 Nov 27. Mol Ecol. 2014. PMID: 24283535 Review. - Molecular evolution and the latitudinal biodiversity gradient.
Dowle EJ, Morgan-Richards M, Trewick SA. Dowle EJ, et al. Heredity (Edinb). 2013 Jun;110(6):501-10. doi: 10.1038/hdy.2013.4. Epub 2013 Mar 13. Heredity (Edinb). 2013. PMID: 23486082 Free PMC article. Review.
Cited by
- Effects of metabolic rate on protein evolution.
Gillooly JF, McCoy MW, Allen AP. Gillooly JF, et al. Biol Lett. 2007 Dec 22;3(6):655-9. doi: 10.1098/rsbl.2007.0403. Biol Lett. 2007. PMID: 17911050 Free PMC article. - Combining palaeontological and neontological data shows a delayed diversification burst of carcharhiniform sharks likely mediated by environmental change.
Brée B, Condamine FL, Guinot G. Brée B, et al. Sci Rep. 2022 Dec 19;12(1):21906. doi: 10.1038/s41598-022-26010-7. Sci Rep. 2022. PMID: 36535995 Free PMC article. - Testing the effect of metabolic rate on DNA variability at the intra-specific level.
McGaughran A, Holland BR. McGaughran A, et al. PLoS One. 2010 Mar 15;5(3):e9686. doi: 10.1371/journal.pone.0009686. PLoS One. 2010. PMID: 20300626 Free PMC article. - Diverse cryptic refuges for life during glaciation.
Pointing SB, Bollard-Breen B, Gillman LN. Pointing SB, et al. Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5452-3. doi: 10.1073/pnas.1403594111. Epub 2014 Apr 7. Proc Natl Acad Sci U S A. 2014. PMID: 24711406 Free PMC article. No abstract available. - On the processes generating latitudinal richness gradients: identifying diagnostic patterns and predictions.
Hurlbert AH, Stegen JC. Hurlbert AH, et al. Front Genet. 2014 Dec 2;5:420. doi: 10.3389/fgene.2014.00420. eCollection 2014. Front Genet. 2014. PMID: 25520738 Free PMC article.
References
- Simpson G. G. Tempo and Mode in Evolution. London: Oxford Univ. Press; 1944.
- Dobzhansky T. Genetics and the Origin of Species. New York: Columbia Univ. Press; 1937.
- Kimura M. The Neutral Theory of Molecular Evolution. Cambridge, U.K.: Cambridge Univ. Press; 1983.
- Coyne J. A., Orr H. A. Speciation. Sunderland, MA: Sinauer; 2004.
- Rohde K. Oikos. 1992;65:514–527.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials