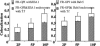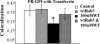Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3'-kinase-dependent machinery - PubMed (original) (raw)
Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3'-kinase-dependent machinery
Manjula Kalia et al. Mol Biol Cell. 2006 Aug.
Abstract
In the process of internalization of molecules from the extracellular milieu, a cell uses multiple endocytic pathways, consequently generating different endocytic vesicles. These primary endocytic vesicles are targeted to specific destinations inside the cell. Here, we show that GPI-anchored proteins are internalized by an Arf6-independent mechanism into GPI-anchored protein-enriched early endosomal compartments (GEECs). Internalized GPI-anchored proteins and the fluid phase are first visualized in GEECs that are acidic, primary endocytic structures, negative for early endosomal markers, Rab4, Rab5, and early endosome antigen (EEA)1. They subsequently acquire Rab5 and EEA1 before homotypic fusion with other GEECs, and heterotypic fusion with endosomes containing cargo from the clathrin-dependent endocytic pathway. Although, the formation of GEECs is unaffected by inhibition of Rab5 GTPase and phosphatidylinositol-3'-kinase (PI3K) activity, their fusion with sorting endosomes is dependent on both activities. Overexpression of Rab5 reverts PI3K inhibition of fusion, providing evidence that Rab5 effectors play important roles in heterotypic fusion between the dynamin-independent GEECs and clathrin- and dynamin-dependent sorting endosomes.
Figures
Figure 1.
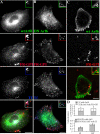
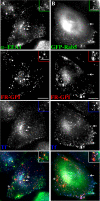
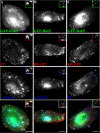
Arf6 is not a specific marker of the GEEC pathway in CHO cells. CHO cells transfected with HA-tagged wt-Arf6 (A and C) or HA-tagged DN-Arf6 (T27N; B) were given a 10-min pulse (A and B) of Cy5-labeled Fab-fragment of anti-folate receptor antibody Mov18 (Cy5-Fab-Mov18; red in A–C) and Cy3-Tf (blue in A and B) or a 2-min pulse of Cy5-Fab-Mov18 (C), fixed, and stained with Alexa 488-labeled anti-Fc-fragment antibody against the HA-tag (green). The cells were imaged on a wide-field microscope (A and B) or a confocal microscope (C) as described in text. Images are presented as gray scales for individual colors, and pseudocolored as indicated before being merged. Insets show a magnified view of the region corresponding to the asterisk (*). Note that Tf-containing endosomes exhibit significant colocalization with Arf6 (pink structures), whereas the GPI-anchored proteins containing endosomes only show an occasional overlap with Arf6 in structures also containing Tf (pinkish white). At 2 min, most of the GEECs are not in any Arf6 structures (C). (D) Histogram showing extent of overlap between Arf6 and Tf endosomes (top l), and Arf6 and FR-GPI endosomes (bottom) at different pulse times (5P, 5 min; and 10P, 10 min). The shaded area enclosed in the open bar indicates the fraction of Tf-positive endosomes (D, top) that colocalized with both Arf6 and FR-GPI, and of FR-GPI-positive endosomes (D, bottom) that colocalized with both Arf6 and Tf. Bar, 10 μm and 5 μm (inset).
Figure 2.
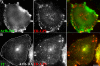
DA-Arf6 does not block fusion between GEECs and sorting endosomes and allows delivery of GPI-anchored proteins to the recycling endosome. (A) CHO cells transfected with HA-tagged DA-Arf6 (green) were given a 10-min pulse of Cy5-Fab-Mov18 (red). Endosomes containing FR-GPI show some overlap with membrane ruffles of DA-Arf6, however a significant fraction of Cy5-Fab-Mov18 labeled endosomes are not colocalized with DA-Arf6. (B) CHO cells transfected with GFP-DA-Arf6 (outlined cell) were given a 20-min pulse of Alexa 568-Tf (green) and Cy5-MOv18 Fab (red). Note fusion of endosomes of Tf and FR-GPI in the periphery of DA-Arf6 expressing cells. FR-GPI and Tf are also delivered to the pericentriolar recycling compartment in transfected cells. Bar, 10 μm.
Figure 3.
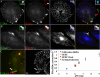
Acidic GEECs are formed from fusion of primary endocytic vesicles. (A and B) CHO cells were pulsed for 2 min at 37°C with FITC-Dex (green) followed by a 30-s wash and a 2-min pulse of TMR-Dex (red), in the presence of Cy5-Tf (blue). Cells were either directly fixed (A) or alternately washed and chased for another 2 min (B) before imaging. Note that the endosomes of FITC-Dex (green, first pulse) are completely separate from those of TMR-Dex (red, second pulse) and are also not colocalized with Tf endosomes (blue; A). After a further chase, fluid endosomes fuse with each other and also with Tf-containing sorting endosomes (B). Insets show a magnified view of the region corresponding to the asterisk (*). Bar, 10 μm and 5 μm (inset). (C) Measurement of endosomal pH. Cells were given a 2-min pulse of FITC-Dex, TMR-Dex, and Cy5-Tf. Note that fluid endosomes that have fused with Tf-containing sorting endosomes (arrow) have a brighter green fluorescence, indicating a higher pH of the endosome compared with fluid endosomes that are not colocalized with Tf (arrowheads). (D) pH calibration. Cell were pulsed for 2 min with a mixture of FITC-Dex and TMR-Dex to label early endosomes, and subsequently equilibrated with a range of different buffer pHs in the presence of 10 μM nigericin to obtain a calibration of the ratio of FITC-Dex to TMR-Dex fluorescence against buffer pHs. This is represented as the ratio of FITC-Dex to TMR-Dex fluorescence normalized to the value at pH 9.0. pH of GEECs (fluid endosomes not colocalized with Tf-containing endosomes; data point in orange) was close of 6, whereas the pH of sorting endosomes (fluid endosomes fused with transferrin; data point in green) was close to 6.5, indicating that GEECs are much more acidic than the sorting endosomes. The experiment was also performed after mild fixation of cells (2 min on ice), with identical results; pH of the GEECs in fixed cells (indicated by data point in red) was also close to 6.
Figure 4.
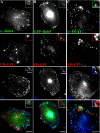
GPI-anchored proteins are internalized into GEECs devoid of early endosome markers. Untransfected CHO cells (A and C) or GFP-Rab5-transfected cells (B) were pulsed with Cy5-Fab-Mov18 (red) and Cy3-Tf (blue) for 2 min at 37°C, fixed, and labeled with antibody against Rab4 (green in A) or EEA1 (green in C) or directly imaged for GFP-fluorescence (green in B). Images of anti-Rab4, GFP-Rab5, or anti-EEA1 (green), FR-GPI (red), Tf (blue) are presented as gray scale images and pseudocolored and color merged (bottom). Insets show a magnified view of the region corresponding to the asterisk (*). Bar, 10 μm and 5 μm (inset).
Figure 5.
GEECs acquire Rab5 and EEA1 before fusion with sorting endosomes. (A and B) Untransfected CHO cells (A) or GFP-Rab5-transfected cells (B) were incubated for 5 min (A) or 10 min (B) with Cy5-Fab-Mov18 (red) and Cy3-Tf (blue) at 37°C, fixed, and labeled with antibody against EEA1 (green in A) or directly imaged for GFP fluorescence (green in B). Images were collected on a wide-field microscope, pseudocolored, and color merged. Insets show a magnified view of the regions corresponding to the asterisk (*). Bar, 10 μm and 5 μm (inset).
Figure 6.
Kinetics of Rab5 and EEA1 loading on GEECs. FR-GPIs expressing cells were incubated with Cy5-Fab-Mov18 and Cy3-Tf and incubated at 37°C for different times (2P, 2 min; 5P, 5 min; and 10P, 10 min). Histogram shows quantitative extent of colocalization of Cy5-Fab-Mov18–positive endosomes with endogenous EEA1 (left) or GFP-Rab5 expressed in the same cells (right). The shaded area enclosed in the open bar indicates the fraction of Cy5-Fab-Mov18–positive endosomes that colocalized with both EEA1 and Tf (left) and with both GFP-Rab5 and Tf (right).
Figure 7.
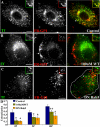
EEA1 loading on GEECs is prevented by PI3K and Rab5 inhibition. CHO cells either untreated (A) or pretreated (B) with 100 nM WT for 30 min. Cells were given a 10-min pulse of Cy5-Fab-Mov18 (red) followed by fixation, permeabilization and staining for EEA1 (green). (C) CHO cells, transfected with DN-GFP-Rab5 (outlined cell in C, left) were pulsed for 5 min with Cy5-Fab-Mov18 (red) followed by fixation, permeabilization, and staining for EEA1 (green). Images were pseudocolored and color combined. Note EEA1 fails to load on 5- or 10-min structures containing endocytosed GPI-anchored proteins. Insets show a magnified view of the regions corresponding to the asterisk (*). Bar, 10 μm and 5 μm (inset).
Figure 8.
Inhibition of PI3K and Rab5 activity block mixing between GEECs and sorting endosomes. Control CHO cells (A), cells pretreated with 100 nM WT (B), or transfected with DN Rab5 (outlined cell in C) were pulsed for 10 min with Cy5-Fab-Mov18 (red) and Cy3-Tf (green), fixed, and imaged. Images were pseudocolored and color combined. Insets show a magnified view of the regions corresponding to the asterisk (*). (D) The extent of colocalization between Cy5-Fab-Mov18-containing (GEECs) and Tf-containing (sorting) endosomes was quantified at different pulse times (2P, 2 min; 5P, 5 min; and 10P, 10 min) for the different treatments as indicated by colored bars (untreated, purple; 100 nM WT, olive green; and DN-Rab5, orange). Colocalization index between FR-GPI and Tf is significantly different between cells treated with 100 nM WT and DN-Rab5 compared with control cells. *p < 0.001 and **p < 0.003, obtained from Student’s t test comparing different data sets. Bar, 10 μm and 5 μm (inset).
Figure 9.
GEEC fuse with early sorting endosome with similar kinetics and in a PI3K-sensitive manner in Cav1−/− mouse embryonic fibroblasts. Mouse embryonic fibroblasts with and without caveolin expression were transfected with CFP-GPI. A 5-min pulse of Alexa 488 CTxB (blue); Alexa 568-anti-GFP-Fab (red), and Cy5-Tf (green) was given to Cav+/+ MEFs (A), Cav−/− MEFs (B), and Cav−/− MEFs (C) pretreated with 100 nM WT for 30 min. Images were pseudocolored, and color combined as described. Insets show a magnified view of the regions corresponding to the asterisk (*). (D) Histogram showing extent of colocalization between GPI and Tf, CTxB and Tf, and CTxB and GPI. Note that a significant fraction of endocytosed CTxB is internalized with CFP-GPI. Bar, 10 μm and 5 μm (inset).
Figure 10.
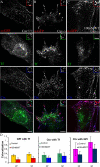
Overexpression of GFP-Rab5 can restore EEA1 loading and rescue fusion in WT-treated cells. CHO cells transfected with GFP-Rab5 (green) were pretreated with 100 nM WT for 30 min followed by pulse of Alexa 568-Tf (red in A, blue in C) and Cy5-Fab-Mov 18 (red in B and C) for 10 min. Cells were fixed, stained for EEA1 (blue in A and B), or directly imaged (C). Images were pseudocolored and color merged as described. Insets show a magnified view of the regions corresponding to the asterisk (*).
Figure 11.
GFP-Rab5 overexpression can rescue fusion between GEECs and sorting endosomes. Histogram shows the extent of colocalization between GEECs and sorting endosomes as described in Materials and Methods. Colocalization index between FR-GPI and Tf in cells transfected with wtRab5, (with and without WT treatment) was not statistically different from control cells; p values ∼0.3, whereas treatment with WT alone was significantly different from control or GFP-Rab5 expressing cells (*p < 0.0001, obtained from Student’s t test by comparing the different data sets. Bar, 10 μm and 5 μm (inset).
Similar articles
- Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes.
Rubino M, Miaczynska M, Lippé R, Zerial M. Rubino M, et al. J Biol Chem. 2000 Feb 11;275(6):3745-8. doi: 10.1074/jbc.275.6.3745. J Biol Chem. 2000. PMID: 10660521 - beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases.
Seachrist JL, Anborgh PH, Ferguson SS. Seachrist JL, et al. J Biol Chem. 2000 Sep 1;275(35):27221-8. doi: 10.1074/jbc.M003657200. J Biol Chem. 2000. PMID: 10854436 - Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis.
Delaney KA, Murph MM, Brown LM, Radhakrishna H. Delaney KA, et al. J Biol Chem. 2002 Sep 6;277(36):33439-46. doi: 10.1074/jbc.M205293200. Epub 2002 Jul 1. J Biol Chem. 2002. PMID: 12093817 - Biogenesis of the sorting endosome: the role of Rab5.
Woodman PG. Woodman PG. Traffic. 2000 Sep;1(9):695-701. doi: 10.1034/j.1600-0854.2000.010902.x. Traffic. 2000. PMID: 11208157 Review. - Endocytosis of lipid-anchored proteins: excluding GEECs from the crowd.
Nichols B. Nichols B. J Cell Biol. 2009 Aug 24;186(4):457-9. doi: 10.1083/jcb.200907119. Epub 2009 Aug 17. J Cell Biol. 2009. PMID: 19687254 Free PMC article. Review.
Cited by
- Endocytosis of glycosylphosphatidylinositol-anchored proteins.
Lakhan SE, Sabharanjak S, De A. Lakhan SE, et al. J Biomed Sci. 2009 Oct 15;16(1):93. doi: 10.1186/1423-0127-16-93. J Biomed Sci. 2009. PMID: 19832981 Free PMC article. Review. - Caveolin-1 induces formation of membrane tubules that sense actomyosin tension and are inhibited by polymerase I and transcript release factor/cavin-1.
Verma P, Ostermeyer-Fay AG, Brown DA. Verma P, et al. Mol Biol Cell. 2010 Jul 1;21(13):2226-40. doi: 10.1091/mbc.e09-05-0417. Epub 2010 Apr 28. Mol Biol Cell. 2010. PMID: 20427576 Free PMC article. - Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation.
Blanchet MH, Le Good JA, Mesnard D, Oorschot V, Baflast S, Minchiotti G, Klumperman J, Constam DB. Blanchet MH, et al. EMBO J. 2008 Oct 8;27(19):2580-91. doi: 10.1038/emboj.2008.174. Epub 2008 Sep 4. EMBO J. 2008. PMID: 18772886 Free PMC article. - Macropinocytosis in Shiga toxin 1 uptake by human intestinal epithelial cells and transcellular transcytosis.
Malyukova I, Murray KF, Zhu C, Boedeker E, Kane A, Patterson K, Peterson JR, Donowitz M, Kovbasnjuk O. Malyukova I, et al. Am J Physiol Gastrointest Liver Physiol. 2009 Jan;296(1):G78-92. doi: 10.1152/ajpgi.90347.2008. Epub 2008 Oct 30. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 18974311 Free PMC article. - Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers.
Lakshminarayan R, Wunder C, Becken U, Howes MT, Benzing C, Arumugam S, Sales S, Ariotti N, Chambon V, Lamaze C, Loew D, Shevchenko A, Gaus K, Parton RG, Johannes L. Lakshminarayan R, et al. Nat Cell Biol. 2014 Jun;16(6):595-606. doi: 10.1038/ncb2970. Epub 2014 May 18. Nat Cell Biol. 2014. PMID: 24837829
References
- Anderson R. G., Kamen B. A., Rothberg K. G., Lacey S. W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
- Bucci P., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in early endocytic pathway. Cell. 1992;70:715–728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources