The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery - PubMed (original) (raw)
The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery
Dominik Oliver et al. J Neurosci. 2006.
Abstract
Large-conductance voltage- and Ca(2+)-activated K+ channels (BKCa) are involved in shaping spiking patterns in many neurons. Less is known about their role in mammalian inner hair cells (IHCs), mechanosensory cells with unusually large BKCa currents. These currents may be involved in shaping the receptor potential, implying crucial importance for the properties of afferent auditory signals. We addressed the function of BKCa by recording sound-induced responses of afferent auditory nerve (AN) fibers from mice with a targeted deletion of the pore-forming alpha-subunit of BKCa (BKalpha(-/-)) and comparing these with voltage responses of current-clamped IHCs. BKCa-mediated currents in IHCs were selectively abolished in BKalpha(-/-), whereas cochlear physiology was essentially normal with respect to cochlear sensitivity and frequency tuning.BKalpha(-/-) AN fibers showed deteriorated precision of spike timing, measured as an increased variance of first spike latency in response to tone bursts. This impairment could be explained by a slowed voltage response in the presynaptic IHC resulting from the reduced K+ conductance in the absence of BKCa. Maximum spike rates of AN fibers were reduced nearly twofold in BKalpha(-/-), contrasting with increased voltage responses of IHCs. In addition to presynaptic changes, which may be secondary to a modest depolarization of BKalpha(-/-) IHCs, this reduction in AN rates suggests a role of BKCa in postsynaptic AN neurons, which was supported by increased refractory periods. In summary, our results indicate an essential role of IHC BKCa channels for precise timing of high-frequency cochlear signaling as well as a function of BKCa in the primary afferent neuron.
Figures
Figure 1.
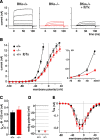
Fast outward K+ currents (_I_K,f) are selectively abolished in IHCs from BKCa knock-out mice. A, K+ currents measured in response to depolarizing voltage steps in nominal increments of 10 mV from a holding potential of −80 mV. Recordings are from a wild-type IHC (BKα+/+; left), from a BKCa knock-out IHC (BKα−/−; middle), and from a BKα+/+ IHC in the presence of the specific BKCa blocker IbTx (100 n
m
; ≥10 min) in the extracellular solution (right). Recordings were made from IHCs in isolated apical turn organs of Corti at 23, 24, and 26 d after birth, respectively. B, Average steady-state current amplitudes from six BKα+/+ and 10 BKα−/− IHCs show a large reduction of overall currents. Residual currents of BKα−/− cells are equal to the IbTx-resistant currents of wild-type IHCs (n = 11). Currents are plotted against membrane potentials corrected for voltage drops across the residual series resistance (horizontal error bars). Inset, Current amplitudes at the physiological voltage range at enlarged scale. C, The background K+ current (_I_K,n) is not different between BKα+/+ (n = 9) and BKα−/− (n = 7) IHCs. Currents are quantified as the maximum deactivating inward tail currents at −120 mV after prepulses to voltages between −150 to −60 mV (Oliver et al., 2003). The voltage dependence of this current was also unchanged (half-activating voltage of −95.6 ± 1.7 and −99.5 ± 3.0 mV, respectively). D, Resting membrane potential in vitro is equal in BKα+/+ (n = 5) and BKα−/− (n = 8) IHCs. Symbol key in B also applies to D and E. E, Calcium currents of IHCs are not affected by ablation of BKCa. Currents through Cav channels from seven BKα+/+ and eight BKα−/− IHCs are measured with 10 m
m
extracellular Ba2+ as the charge carrier and with intracellular Cs+/4-AP and extracellular tetraethylammonium (10 m
m
) to block all K+ currents.
Figure 2.
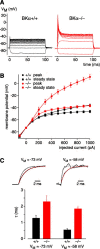
Voltage responses of _BKα_−/− IHCs have increased amplitudes and slowed time constants. A, Voltage responses measured from current-clamped IHCs show increased onset and steady-state amplitudes in BKα−/− IHCs. Voltage responses were elicited by current steps in 100 pA increments. B, Average peak and steady-state voltage responses measured from five BKα+/+ and seven BKα−/− IHCs as in A are displayed as a function of the current step. Correspondence between genotype and symbol is shown in the key. C, Top, Onset of voltage responses of IHCs to a 100 pA current step recorded from BKα+/+ IHCs (gray) and BKα−/− IHCs (red). Recordings were obtained from either resting potential (left) or cells held close to −58 mV, a voltage probably similar to the in vivo resting potential (right; see Results). Non-averaged voltage traces are plotted normalized to maximum. Time constants are derived from monoexponential fits (dashed lines) to the voltage onsets. Bottom, Time constants were significantly slower in BKα−/− IHCs than in BKα+/+, both for the in vitro resting potential (n = 7 and 5 IHCs for BKα−/− and BKα+/+, respectively; p < 0.05) and at −58 mV (n = 3 each; p < 0.001).
Figure 3.
Thresholds in the BKα−/− mice are slightly elevated, but tuning properties of AN fibers are not altered. A, Cochlear sensitivity was measured via isoresponse contours for AN fibers. Thresholds show a large overlap between individual AN fibers of wild-type and BKα−/− ears with a slight elevation of the average threshold in BKα−/−. As seen in the inset, each point in this scatter plot represents threshold at the characteristic frequency of an AN. B, Sharpness of tuning of AN fibers shows no differences between BKα−/− versus BKα+/+ ears. Tuning is measured as “Q10 dB,” i.e., the characteristic frequency divided by the bandwidth at 10 dB above threshold. Symbol key in A also applies to B.
Figure 4.
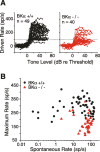
AN fibers from BKα−/− ears show a decrease in saturated discharge rates. A, All rate-versus-level functions for AN fibers with high spontaneous discharge rates (>1 sp/s) are superimposed for BKα+/+ (black) and BKα−/− (red) ears. The position of each function along the _x_-axis is normalized to the model-fit threshold (see Materials and Methods). The “driven rate” (_y_-axis) is defined as the average discharge rate minus the spontaneous rate. B, Maximum rates are plotted versus SR for all fibers from BKα−/− and BKα+/+ ears. Fibers with spontaneous rate of 0 are plotted as 0.05 sp/s. Correspondence between symbol type and genotype is shown in the key. Maximum rates are derived from the model-fit data (see Materials and Methods) and are larger than the driven-rate values in A, because spontaneous rates have not been subtracted out.
Figure 5.
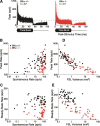
Both onset rate and steady-state rate are diminished in the AN of BKα−/− mice. A, Poststimulus time histograms for responses to tone bursts at the CF superimposed from all AN fibers with high spontaneous rates (>1 sp/s) from the genotypes indicated. The tone-burst stimuli were 50 ms in duration. Dashed line indicates mean steady-state rates from wild-type fibers. B, Peak rates were calculated as the maximum value from the poststimulus time histogram divided by the bin width (0.5 ms). Fibers with spontaneous rate of 0 were plotted as 0.05 sp/s. C, Steady-state rate was calculated as the average rate for the final 15 ms of the tone burst. D, E, The variance of the FSL was extracted from the distribution of spike times for the first spike discharge after each tone-burst onset. Peak and steady-state rates are measured as described for B and C, respectively. The analyses of spike rates and spike timing in this figure are based on spike times measured in response to 200–300 tone bursts at the characteristic frequency, presented 30 dB above threshold.
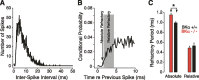
Figure 6.
The absolute refractory period is prolonged in AN fibers of BKα−/− mice. Estimates of absolute and relative refractory periods were extracted from conditional probability histograms (Li and Young, 1993) based on spike times seen in response to tone bursts at the characteristic frequency. A, First, interspike interval histograms were computed using only spikes occurring during the steady-state response (>20 ms after tone burst onset). B, The “hazard function” plots the conditional probability of discharge as a function of the time since the previous spike. For additional details, see text. C, Mean ± SEM values of absolute and relative refractory periods for all AN fibers sampled in the three groups of mice. The difference between absolute refractory periods in BKα−/− and BKα+/+ was highly significant (*p = 0.00002, two-tailed t test).
Similar articles
- Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels.
Nemzou N RM, Bulankina AV, Khimich D, Giese A, Moser T. Nemzou N RM, et al. Neuroscience. 2006 Sep 15;141(4):1849-60. doi: 10.1016/j.neuroscience.2006.05.057. Epub 2006 Jul 10. Neuroscience. 2006. PMID: 16828974 - The Ca2+ channel subunit beta2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing.
Neef J, Gehrt A, Bulankina AV, Meyer AC, Riedel D, Gregg RG, Strenzke N, Moser T. Neef J, et al. J Neurosci. 2009 Aug 26;29(34):10730-40. doi: 10.1523/JNEUROSCI.1577-09.2009. J Neurosci. 2009. PMID: 19710324 Free PMC article. - Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the Guinea pig cochlea.
Skinner LJ, Enée V, Beurg M, Jung HH, Ryan AF, Hafidi A, Aran JM, Dulon D. Skinner LJ, et al. J Neurophysiol. 2003 Jul;90(1):320-32. doi: 10.1152/jn.01155.2002. Epub 2003 Feb 12. J Neurophysiol. 2003. PMID: 12611976 - Voltage-gated K(+) channels contributing to temporal precision at the inner hair cell-auditory afferent nerve fiber synapses in the mammalian cochlea.
Oak MH, Yi E. Oak MH, et al. Arch Pharm Res. 2014 Jul;37(7):821-33. doi: 10.1007/s12272-014-0411-8. Epub 2014 Jun 14. Arch Pharm Res. 2014. PMID: 24925343 Review. - Cochlear processes reflected in responses of the cochlear nerve.
Smith RL. Smith RL. Acta Otolaryngol. 1985 Jul-Aug;100(1-2):1-12. doi: 10.3109/00016488509108580. Acta Otolaryngol. 1985. PMID: 2992224 Review.
Cited by
- An Anatomical and Physiological Basis for Flexible Coincidence Detection in the Auditory System.
Kreeger LJ, Honnuraiah S, Maeker S, Shea S, Fishell G, Goodrich LV. Kreeger LJ, et al. bioRxiv [Preprint]. 2024 Sep 3:2024.02.29.582808. doi: 10.1101/2024.02.29.582808. bioRxiv. 2024. PMID: 38464181 Free PMC article. Preprint. - Bile Acid Application in Cell-Targeting for Molecular Receptors in Relation to Hearing: A Comprehensive Review.
Ionescu CM, Jones MA, Wagle SR, Kovacevic B, Foster T, Mikov M, Mooranian A, Al-Salami H. Ionescu CM, et al. Curr Drug Targets. 2024;25(3):158-170. doi: 10.2174/0113894501278292231223035733. Curr Drug Targets. 2024. PMID: 38192136 Review. - Hyperacusis in the Adult _Fmr1_-KO Mouse Model of Fragile X Syndrome: The Therapeutic Relevance of Cochlear Alterations and BKCa Channels.
Ferraguto C, Bouleau Y, Peineau T, Dulon D, Pietropaolo S. Ferraguto C, et al. Int J Mol Sci. 2023 Jul 24;24(14):11863. doi: 10.3390/ijms241411863. Int J Mol Sci. 2023. PMID: 37511622 Free PMC article. - Small molecule modulation of the large-conductance calcium-activated potassium channel suppresses salicylate-induced tinnitus in mice.
Scott LL, Lowe AS, Brecht EJ, Franco-Waite L, Walton JP. Scott LL, et al. Front Neurosci. 2022 Aug 25;16:763855. doi: 10.3389/fnins.2022.763855. eCollection 2022. Front Neurosci. 2022. PMID: 36090293 Free PMC article. - Synaptic Release Potentiation at Aging Auditory Ribbon Synapses.
Peineau T, Belleudy S, Pietropaolo S, Bouleau Y, Dulon D. Peineau T, et al. Front Aging Neurosci. 2021 Oct 18;13:756449. doi: 10.3389/fnagi.2021.756449. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34733152 Free PMC article.
References
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL (2002). Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol 447:331–350. - PubMed
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B (1999). Molecular diversity of K+ channels. Ann NY Acad Sci 868:233–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous