Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport - PubMed (original) (raw)
Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport
Tim T Lambers et al. EMBO J. 2006.
Abstract
In Ca(2+)-transporting epithelia, calbindin-D(28K) (CaBP(28K)) facilitates Ca(2+) diffusion from the luminal Ca(2+) entry side of the cell to the basolateral side, where Ca(2+) is extruded into the extracellular compartment. Simultaneously, CaBP(28K) provides protection against toxic high Ca(2+) levels by buffering the cytosolic Ca(2+) concentration ([Ca(2+)](i)) during high Ca(2+) influx. CaBP(28K) consistently colocalizes with the epithelial Ca(2+) channel TRPV5, which constitutes the apical entry step in renal Ca(2+)-transporting epithelial cells. Here, we demonstrate using protein-binding analysis, subcellular fractionation and evanescent-field microscopy that CaBP(28K) translocates towards the plasma membrane and directly associates with TRPV5 at a low [Ca(2+)](i). (45)Ca(2+) uptake measurements, electrophysiological recordings and transcellular Ca(2+) transport assays of lentivirus-infected primary rabbit connecting tubule/distal convolute tubule cells revealed that associated CaBP(28K) tightly buffers the flux of Ca(2+) entering the cell via TRPV5, facilitating high Ca(2+) transport rates by preventing channel inactivation. In summary, CaBP(28K) acts in Ca(2+)-transporting epithelia as a dynamic Ca(2+) buffer, regulating [Ca(2+)] in close vicinity to the TRPV5 pore by direct association with the channel.
Figures
Figure 1
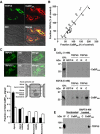
Coordinated expression and direct association of TRPV5 and CaBP28K. (A) Colocalization of TRPV5 (green) and CaBP28K (red) in the DCT and CNT. (B) Correlation in TRPV5 and CaBP28K expression after treatment (1parathyroidectomized (van Abel et al, 2005); 2tacrolimus (Nijenhuis et al, 2005); 3acidose/alkalose (Nijenhuis et al, 2006); 4thiazide (Nijenhuis et al, 2004, 2005); 5calcimemetics (van Abel et al, 2005); 6ovariectomized (van Abel et al, 2005); 7dexamethasone (Nijenhuis et al, 2004); 8ovariectomized+vitamine D3 (van Abel et al, 2002)). _R_2=0.9068. (C) Localization of CaBP28K in kidney sections of TRPV5−/− and wild-type mice. The epithelial cells were divided in different regions including apical, apical-middle, middle, basolateral-middle and basolateral as indicated. The immuno-positive CaBP28K staining of these different cellular regions in 30 cells of six different tubules was calculated as described in Materials and methods. Significant differences in CaBP28K intensities within the group are indicated by an asterisk. Open bars represent TRPV5%, closed bars represent wild-type. (D) [35S]Methionine-labeled, _in vitro_-translated CaBP28K was incubated either in the presence (1 mM CaCl2) or absence (5 mM EDTA) of Ca2+, with GST or GST fused to the N- or C-terminus of TRPV5 and TRPV6 immobilized on glutathione-Sepharose 4B beads. Input control (IP) represents 10% of the total pull-down input. (E) Cells were cotransfected with pEBG-CaBP28K (GST-CaBP28K) and pCINeo-TRPV5-IRES-EGFP or pEBG (GST) and pCINeo-TRPV5-IRES-EGFP (control, C). To decrease the [Ca2+]i, cells were treated with BAPTA-AM. Lysates were loaded on glutathione-Sepharose 4B beads, and after extensive washing co-precipitation was investigated by immunoblotting using the guinea-pig anti-TRPV5 antibody (IP=input). The two TRPV5 immuno-positive bands correspond to the core (lower) and glycosylated forms of the protein.
Figure 2
Subcellular localization of CaBP28K at low intracellular Ca2+ concentrations. (A) Cells were transfected with CaBP28K and TRPV5 (left panel) or CaBP28K and empty vector (right panel) and treated with or without BAPTA-AM. Plasma membrane-enriched fractions were probed for the presence of endogenously expressed Na,K-ATPase and exogenously expressed CaBP28K using anti-Na,K-ATPase and anti-CaBP28K antibodies, respectively. NT=non-transfected. (B) Plasma membrane-enriched fractions of primary CNT/CCD cultures, either treated with or without BAPTA-AM, were isolated and probed for the presence of endogenously expressed CaBP28K and Na,K-ATPase. Representative blots of three independent experiments are shown.
Figure 3
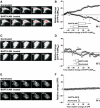
CaBP28K translocation at low intracellular Ca2+ concentrations. (A) TIRF images of a single cell expressing EYFP-CaBP28K and TRPV5-ECFP that was treated with (lower panel) or without (upper panel) BAPTA-AM. Scale bar=5 μm. (B) Average time courses of the TIRF signal of EYFP-CaBP28K in EYFP-CaBP28K- and TRPV5-ECFP-expressing cells that were treated either with (▪) or without (Δ) BAPTA-AM (_n_=7). Significant differences in total EYFP changes after BAPTA-AM treatment (inset) are indicated by an asterisk (P<0.05). (C) TIRF images of a single cell expressing EYFP-CaBP28K that was treated with (lower panel) or without (upper panel) BAPTA-AM. Scale bar=5 μm. (D) Average time courses of the TIRF signal of EYFP-CaBP28K-expressing cells that were treated either with (▪) or without (Δ) BAPTA-AM (_n_=5). (E) TIRF images of single cells expressing TRPV5-IRES-EGFP treated with (lower panel) or without (upper panel) BAPTA-AM. Scale bar=5 μm. (F) Average time courses of the TIRF signal in TRPV5-IRES-EGFP-expressing cells that were treated either with (▪) or without (Δ) BAPTA-AM (_n_=5). In all images, a gradient filter was applied such that saturation of TIRF fluorescence turns red and the intensities were measured between 5 and 10% of the visible ‘footprint' of the cell.
Figure 4
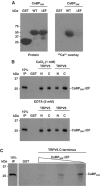
Characterization of a Ca2+-insensitive CaBP28K mutant. (A) Wild-type CaBP28K and CaBP28KΔEF were fused to GST (left panel) and 45Ca2+ binding was determined (right panel). (B) [35S]Methionine-labeled, _in vitro_-translated CaBP28KΔEF was incubated either in the presence (1 mM CaCl2) or absence (5 mM EDTA) of Ca2+, with GST or GST fused to the N- and C-termini of TRPV5 and TRPV6 immobilized on glutathione-Sepharose 4B beads. Input control (IP) represents 10% of the total pull-down input. (C) [35S]Methionine-labeled _in vitro_-translated CaBP28K was incubated in the presence of increasing amounts of non-radioactive _in vitro_-translated CaBP28KΔEF with GST or GST fused to the C-termini of TRPV5 immobilized on glutathione-Sepharose 4B beads. This pull-down experiment was performed in the absence of Ca2+ (5 mM EDTA). Input control (IP) represents 10% of the input.
Figure 5
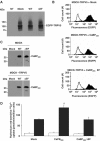
Role of CaBP28K in TRPV5-mediated 45Ca2+ uptake. EGFP-TRPV5 and CaBP28K, CaBP28KΔEF or empty vector were stably expressed in MDCK cells. The expression level of EGFP-tagged TRPV5 as determined using rabbit anti-GFP antibody (A) or flow cytometry analysis (B; black peaks) reveals that the expression of TRPV5 in empty vector- and CaBP28K-expressing cells is equal. The open peak in the three panels indicates background fluorescence in non-transfected cells. The two TRPV5 immuno-positive bands correspond to the core (lower) and glycosylated forms of EGFP-TRPV5. (C) The stable expression of CaBP28K and CaBP28KΔEF in MDCK cells or MDCK cells expressing EGFP-TRPV5 was verified using anti-CaBP28K antibodies. (D) Ruthenium red-sensitive 45Ca2+ uptake of MDCK cells stably expressing both (black bars) TRPV5 and CaBP28K, CaBP28KΔEF or empty vector and MDCK cells stably expressing (open bars) CaBP28K, CaBP28KΔEF or empty vector. Significant differences in 45Ca2+ uptake are indicated by an asterisk (P<0.05).
Figure 6
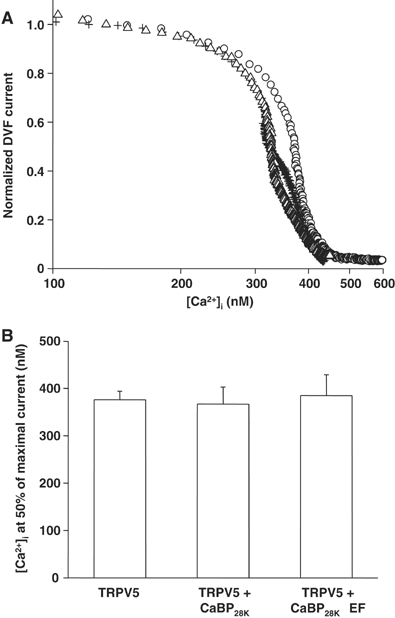
Direct influence of CaBP28K on the characteristics of TRPV5. (A) Dose–response curves showing the effect of an increasing [Ca2+]i on the normalized Na+ inward current in divalent-free extracellular solution (DVF) of cells expressing TRPV5 (O, _n_=9), TRPV5 and CaBP28K (+, _n_=11) or TRPV5 and CaBP28KΔEF (Δ, _n_=12). (B) Averaged IC50 for each of the transfections and recordings shown in panel A.
Figure 7
Effect of CaBP28KΔEF on transcellular Ca2+ transport. (A) Effect of lentivirus-mediated overexpression of GFP and GFP-CaBP28KΔEF on transcellular Ca2+ transport in primary rabbit CNT/CCD cells. Averaged transcellular Ca2+ transport for each infection is expressed as mean±s.e.m. A 10 μM portion of ruthenium red was added to the apical side of the cell monolayer during the transport assay to estimate the TRPV5-mediated Ca2+ transport. Significant differences as compared to mock-infected cells are indicated by an asterisk (P<0.05). (B) The expression of GFP and GFP-CaBP28KΔEF (upper panel) was assessed with immunoblotting using rabbit anti-GFP antibody and the expression of endogenous CaBP28K was checked with monoclonal anti-CaBP28K antibody that does not recognize GFP-CaBP28KΔEF (lower panel). To check for equal loading, the expression of the endogenously expressed Na,K-ATPase was measured (lower panel).
Similar articles
- Phenotype of a calbindin-D9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model.
Lee GS, Lee KY, Choi KC, Ryu YH, Paik SG, Oh GT, Jeung EB. Lee GS, et al. J Bone Miner Res. 2007 Dec;22(12):1968-78. doi: 10.1359/jbmr.070801. J Bone Miner Res. 2007. PMID: 17696760 - Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5.
Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ. Topala CN, et al. Cell Calcium. 2009 Apr;45(4):331-9. doi: 10.1016/j.ceca.2008.12.003. Epub 2009 Jan 20. Cell Calcium. 2009. PMID: 19157541 - Effect of dietary calcium and 1,25-(OH)2D3 on the expression of calcium transport genes in calbindin-D9k and -D28k double knockout mice.
Ko SH, Choi KC, Oh GT, Jeung EB. Ko SH, et al. Biochem Biophys Res Commun. 2009 Feb 6;379(2):227-32. doi: 10.1016/j.bbrc.2008.12.029. Epub 2008 Dec 25. Biochem Biophys Res Commun. 2009. PMID: 19100715 - Concerted action of associated proteins in the regulation of TRPV5 and TRPV6.
Schoeber JP, Hoenderop JG, Bindels RJ. Schoeber JP, et al. Biochem Soc Trans. 2007 Feb;35(Pt 1):115-9. doi: 10.1042/BST0350115. Biochem Soc Trans. 2007. PMID: 17233615 Review. - Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1alpha,25-dihydroxy Vitamin D3 and dietary Ca2+.
van de Graaf SF, Boullart I, Hoenderop JG, Bindels RJ. van de Graaf SF, et al. J Steroid Biochem Mol Biol. 2004 May;89-90(1-5):303-8. doi: 10.1016/j.jsbmb.2004.03.029. J Steroid Biochem Mol Biol. 2004. PMID: 15225790 Review.
Cited by
- Alteration of calcium homeostasis in primary preeclamptic syncytiotrophoblasts: effect on calcium exchange in placenta.
Haché S, Takser L, LeBellego F, Weiler H, Leduc L, Forest JC, Giguère Y, Masse A, Barbeau B, Lafond J. Haché S, et al. J Cell Mol Med. 2011 Mar;15(3):654-67. doi: 10.1111/j.1582-4934.2010.01039.x. J Cell Mol Med. 2011. PMID: 20178461 Free PMC article. - What structures did, and did not, reveal about the function of the epithelial Ca2+ channels TRPV5 and TRPV6.
Rohacs T, Fluck EC, De Jesús-Pérez JJ, Moiseenkova-Bell VY. Rohacs T, et al. Cell Calcium. 2022 Sep;106:102620. doi: 10.1016/j.ceca.2022.102620. Epub 2022 Jul 3. Cell Calcium. 2022. PMID: 35834842 Free PMC article. Review. - Calcium-sensing receptor in cancer: good cop or bad cop?
Chakravarti B, Dwivedi SK, Mithal A, Chattopadhyay N. Chakravarti B, et al. Endocrine. 2009 Jun;35(3):271-84. doi: 10.1007/s12020-008-9131-5. Epub 2008 Nov 15. Endocrine. 2009. PMID: 19011996 Review. - Transepithelial calcium transport in prolactin-exposed intestine-like Caco-2 monolayer after combinatorial knockdown of TRPV5, TRPV6 and Ca(v)1.3.
Nakkrasae LI, Thongon N, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Nakkrasae LI, et al. J Physiol Sci. 2010 Jan;60(1):9-17. doi: 10.1007/s12576-009-0068-0. Epub 2009 Nov 3. J Physiol Sci. 2010. PMID: 19885716 Free PMC article. - The renaissance of Ca2+-binding proteins in the nervous system: secretagogin takes center stage.
Alpár A, Attems J, Mulder J, Hökfelt T, Harkany T. Alpár A, et al. Cell Signal. 2012 Feb;24(2):378-387. doi: 10.1016/j.cellsig.2011.09.028. Epub 2011 Oct 1. Cell Signal. 2012. PMID: 21982882 Free PMC article. Review.
References
- Berggard T, Miron S, Onnerfjord P, Thulin E, Akerfeldt KS, Enghild JJ, Akke M, Linse S (2002) Calbindin D28K exhibits properties characteristic of a Ca2+ sensor. J Biol Chem 277: 16662–16672 - PubMed
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE (2004) Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6: 709–720 - PubMed
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A (2003) Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28K-containing terminals. Neuron 38: 79–88 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous