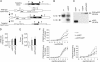Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo - PubMed (original) (raw)
Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo
Grégory Scherrer et al. Proc Natl Acad Sci U S A. 2006.
Abstract
The combination of fluorescent genetically encoded proteins with mouse engineering provides a fascinating means to study dynamic biological processes in mammals. At present, green fluorescent protein (GFP) mice were mainly developed to study gene expression patterns or cell morphology and migration. Here we used enhanced GFP (EGFP) to achieve functional imaging of a G protein-coupled receptor (GPCR) in vivo. We created mice where the delta-opioid receptor (DOR) is replaced by an active DOR-EGFP fusion. Confocal imaging revealed detailed receptor neuroanatomy throughout the nervous system of knock-in mice. Real-time imaging in primary neurons allowed dynamic visualization of drug-induced receptor trafficking. In DOR-EGFP animals, drug treatment triggered receptor endocytosis that correlated with the behavioral response. Mice with internalized receptors were insensitive to subsequent agonist administration, providing evidence that receptor sequestration limits drug efficacy in vivo. Direct receptor visualization in mice is a unique approach to receptor biology and drug design.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
DOR-EGFP knockin mice. (A) Targeting strategy. Oprd1 exons, EGFP cDNA, and the floxed (triangles) hygromycine cassette are displayed as empty, gray, and black boxes, respectively. Homologous recombination (HR) was followed by Cre recombinase treatment (Cre) in ES cells. (B) Southern blot analysis of BamHI-digested genomic DNA from positive ES cells (right lane) by using probe shown in A (thick bar). (C) Western blot analysis of brain membrane preparations from wild-type (Oprd1 +/+), heterozygous (Oprd1 +/EGFP) and homozygous (Oprd1 EGFP/EGFP) animals by using an anti-GFP antibody, with an extract of EGFP-transfected COS cell (derived from African green monkey kidney) as a control (left lane). (D) Quantification of Oprd1 transcription on RNA preparations from brains (n = 8). (E) Number of DOR binding sites in brain membrane preparations (n = 7). (F) Agonist-induced G protein activation in brain membrane preparations (SNC80, n = 6; Met-enkephalin, n = 4; deltorphin II, n = 8). One, two, and three asterisks correspond to P values for genotype effect <0.05, 0.01, and 0.001, respectively.
Fig. 2.
Anatomical distribution of fluorescence in DOR-EGFP mice. (A) Macroscopic view of whole brain (Left), as well as coronal and sagittal brain sections of Oprd1 EGFP/EGFP mice (Right). (B) Confocal images of hippocampus (Hip), basolateral amygdala (Bla), olfactory bulb (Ob), and caudate putamen (Cpu), from areas indicated in A (Insets). (Scale bar: 70 μm.) (C) Primary neurons from Cpu and Hip. (Scale bar: 14 μm.)
Fig. 3.
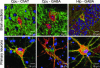
Coexpression of DOR-EGFP (green) with Chat or GABA (red) in brain sections (Upper) and primary neurons (Lower) of caudate putamen (Cpu) and hippocampus (Hip). Cell nuclei are labeled with DAPI (blue). Merged, representative images are shown, and yellow indicates DOR-EGFP colocalization with either Chat or GABA. Quantification indicates that 76% of Chat+ neurons are DOR-EGFP+ in Cpu (n = 19) and in primary Cpu cultures (n = 741). Also 13% and 11% of GABA+ cells express DOR-EGFP in Cpu (n = 31) and Cpu cultures (n = 2526), respectively. In Hip and in primary Hip cultures, 13% (n = 21) and 25% (n = 661) GABAergic neurons are DOR-EGFP+, respectively.
Fig. 4.
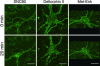
Real-time confocal imaging of SNC80 (100 nM), deltorphin II (100 nM), and Met-enkephalin (1 μM)-induced DOR-EGFP redistribution in primary caudate putamen neurons (see Movies 2, 3, and 4). A representative experiment is shown (n = 6, 5, and 4 for SNC80, deltorphin II, and Met-enkephalin, respectively, referring to separate cell cultures from pools of 6 to 8 mouse pups). Images at 0 and 20 min of treatment, extracted from the corresponding movies, are displayed. (Scale bar: 12 μm.)
Fig. 5.
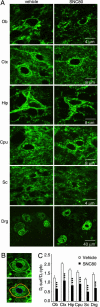
DOR-EGFP endocytosis in vivo. (A) Confocal imaging of DOR-EGFP in neurons of olfactory bulb (Ob), cortex (Ctx), hippocampus (Hip), caudate putamen (Cpu), spinal cord (Sc), and dorsal root ganglia (Drg), 20 min after vehicle (Left) or SNC80 (10 mg/kg, s.c.) (Right) treatment of DOR-EGFP knockin mice. A representative image is shown from two mice per treatment (B) One example for quantifying subcellular fluorescence at the surface and in cytoplasmic compartments of neuron cell bodies, delimited by red/yellow and yellow/blue lines, respectively (see Methods). (C) Quantification of DOR-EGFP internalization from experiment shown in A and expressed as ratio of surface (Df surf) versus cytoplasmic (Df cyto) fluorescence densities (n = 10 per area per treatment). Three asterisks correspond to P value for treatment effect <0.001.
Fig. 6.
DOR-EGFP endocytosis and locomotor response. (A) Basal locomotor activity (n = 24 per group). (B) Total locomotor activity in Oprd1 +/+, EGFP/EGFP, and −/− mice over 2 h, after vehicle (V) or SNC80 (0.3, 1 and 3 mg/kg) administration (n = 10–12 per group). (C) Confocal imaging of DOR-EGFP in caudate putament neurons 2 h after treatments performed in B. (D) Quantification of DOR-EGFP endocytosis from experiment shown in C (n = 10 per group). (E) Negative correlation between surface receptors density (Df surf/Df cyto) and locomotor activity. (F) Locomotor response in EGFP/EGFP mice, in response to a first injection (black arrow) of vehicle (V) or SNC80 (0.3, 1, and 3 mg/kg) and to a second injection (gray arrow) of SNC80 (3 mg/kg) (n = 10 per group). (G) Total locomotor activity in the second 2-h period for the four experimental groups shown in F, compared with vehicle-treated animals (V). One, two, and three asterisks correspond to P values for treatment effect <0.05, 0.01, and 0.001, respectively.
Similar articles
- Mu and Delta Opioid Receptors Are Coexpressed and Functionally Interact in the Enteric Nervous System of the Mouse Colon.
DiCello JJ, Carbone SE, Saito A, Rajasekhar P, Ceredig RA, Pham V, Valant C, Christopoulos A, Veldhuis NA, Canals M, Massotte D, Poole DP. DiCello JJ, et al. Cell Mol Gastroenterol Hepatol. 2020;9(3):465-483. doi: 10.1016/j.jcmgh.2019.11.006. Epub 2019 Nov 20. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31759144 Free PMC article. - In vivo delta opioid receptor internalization controls behavioral effects of agonists.
Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gavériaux-Ruff C, Kieffer BL. Pradhan AA, et al. PLoS One. 2009;4(5):e5425. doi: 10.1371/journal.pone.0005425. Epub 2009 May 1. PLoS One. 2009. PMID: 19412545 Free PMC article. - In vivo techniques to investigate the internalization profile of opioid receptors.
Pradhan AA, Tawfik VL, Tipton AF, Scherrer G. Pradhan AA, et al. Methods Mol Biol. 2015;1230:87-104. doi: 10.1007/978-1-4939-1708-2_7. Methods Mol Biol. 2015. PMID: 25293318 Free PMC article. - Mechanism and consequences of delta-opioid receptor internalization.
Eisinger DA, Schulz R. Eisinger DA, et al. Crit Rev Neurobiol. 2005;17(1):1-26. doi: 10.1615/critrevneurobiol.v17.i1.10. Crit Rev Neurobiol. 2005. PMID: 16307525 Review. - The delta opioid receptor tool box.
Vicente-Sanchez A, Segura L, Pradhan AA. Vicente-Sanchez A, et al. Neuroscience. 2016 Dec 3;338:145-159. doi: 10.1016/j.neuroscience.2016.06.028. Epub 2016 Jun 24. Neuroscience. 2016. PMID: 27349452 Free PMC article. Review.
Cited by
- Chronic Morphine Reduces Surface Expression of δ-Opioid Receptors in Subregions of Rostral Striatum.
Leah PM, Heath EM, Balleine BW, Christie MJ. Leah PM, et al. Neurochem Res. 2016 Mar;41(3):500-9. doi: 10.1007/s11064-015-1638-6. Epub 2015 Jun 21. Neurochem Res. 2016. PMID: 26093651 - Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus.
Erbs E, Faget L, Scherrer G, Kessler P, Hentsch D, Vonesch JL, Matifas A, Kieffer BL, Massotte D. Erbs E, et al. Neuroscience. 2012 Sep 27;221:203-13. doi: 10.1016/j.neuroscience.2012.06.023. Epub 2012 Jun 28. Neuroscience. 2012. PMID: 22750239 Free PMC article. - Mu and Delta Opioid Receptors Are Coexpressed and Functionally Interact in the Enteric Nervous System of the Mouse Colon.
DiCello JJ, Carbone SE, Saito A, Rajasekhar P, Ceredig RA, Pham V, Valant C, Christopoulos A, Veldhuis NA, Canals M, Massotte D, Poole DP. DiCello JJ, et al. Cell Mol Gastroenterol Hepatol. 2020;9(3):465-483. doi: 10.1016/j.jcmgh.2019.11.006. Epub 2019 Nov 20. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31759144 Free PMC article. - Reward processing by the opioid system in the brain.
Le Merrer J, Becker JA, Befort K, Kieffer BL. Le Merrer J, et al. Physiol Rev. 2009 Oct;89(4):1379-412. doi: 10.1152/physrev.00005.2009. Physiol Rev. 2009. PMID: 19789384 Free PMC article. Review. - Dynamics of somatostatin type 2A receptor cargoes in living hippocampal neurons.
Lelouvier B, Tamagno G, Kaindl AM, Roland A, Lelievre V, Le Verche V, Loudes C, Gressens P, Faivre-Baumann A, Lenkei Z, Dournaud P. Lelouvier B, et al. J Neurosci. 2008 Apr 23;28(17):4336-49. doi: 10.1523/JNEUROSCI.4379-07.2008. J Neurosci. 2008. PMID: 18434512 Free PMC article.
References
- Pierce K. L., Premont R. T., Lefkowitz R. J. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. - PubMed
- Ferguson S. S. Pharmacol. Rev. 2001;53:1–24. - PubMed
- von Zastrow M. Life Sci. 2003;74:217–224. - PubMed
- Kieffer B. L., Gavériaux-Ruff C. Prog. Neurobiol. 2002;66:285–306. - PubMed
- Ahmad S., Dray A. Curr. Opin. Investig. Drugs. 2004;5:67–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials