The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange - PubMed (original) (raw)
The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange
Parth Patwari et al. J Biol Chem. 2006.
Abstract
The thioredoxin system plays an important role in maintaining a reducing environment in the cell. Recently, several thioredoxin binding partners have been identified and proposed to mediate aspects of redox signaling, but the significance of these interactions is unclear in part due to incomplete understanding of the mechanism for thioredoxin binding. Thioredoxin-interacting protein (Txnip) is critical for regulation of glucose metabolism, the only currently known function of which is to bind and inhibit thioredoxin. We explored the mechanism of the Txnip-thioredoxin interaction and present evidence that Txnip and thioredoxin form a stable disulfide-linked complex. We identified two Txnip cysteines that are important for thioredoxin binding and showed that this interaction is consistent with a disulfide exchange reaction between oxidized Txnip and reduced thioredoxin. These cysteines are not conserved in the broader family of arrestin domain-containing proteins, and we demonstrate that the thioredoxin-binding property of Txnip is unique. These data suggest that Txnip is a target of reduced thioredoxin and provide insight into the potential role of Txnip as a redox-sensitive signaling protein.
Figures
Figure 1
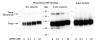
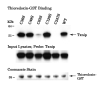
Detection of the thioredoxin-Txnip complex by nonreducing gel electrophoresis. Txnip was overexpressed in 293 cells and the lysates were incubated overnight with thioredoxin-C73S-GST beads. After three washes in lysis buffer, the complex was released by incubation in nonreducing sample buffer at 80 ° for 10 min. The supernatant was divided and dithiothreitiol (DTT) was added to 0, 0.1, 1, or 10 mM. The original lysates and the bound protein samples were then subject to SDS-PAGE and Western analysis for Txnip with the monoclonal antibody JY2. The experiment was replicated with essentially identical results as those shown.
Figure 2
The arrestin-domain-containing (Arrdc) family of proteins. (A) In addition to Txnip, at least five arrestin-domain-containing proteins of unknown function are present in the mouse and human genomes. All have predicted structural homology to the arrestins and contain both N-terminal and C-terminal arrestin domains. Percentage identity to Txnip is shown. (B) Among the three proteins with highest homology to Txnip (Arrdc2, 3, and 4), alignment of the predicted protein sequences revealed that only one cysteine (Txnip C267) is conserved.
Figure 3
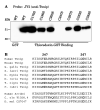
Binding of thioredoxin-GST beads to Txnip Cys-to-Ser mutants. (A) Wildtype and mutant Txnip proteins were overexpressed in 293 cells and the cell lysates were incubated with thioredoxin-GST beads. Wildtype Txnip was incubated with GST alone as a negative control. Bound Txnip was detected by Western analysis with JY2. Only Txnip C247S did not bind to thioredoxin-GST. The experiment was replicated with essentially identical results as those shown. (B) Alignment of Txnip homologs demonstrated that C247 is relatively conserved in vertebrate Txnip orthologs (shown are sequences from Gallus gallus, Xenopus laevis, Oncorhynchus mykiss, Danio rerio, Tetraodon nigroviridus, and Fugu rubripes) but not present in other Arrdc proteins and invertebrate arrestin-domain-containing proteins (shown are sequences from Caenorhabditis elegans and Drosophila melanogaster) despite conservation of C267.
Figure 4
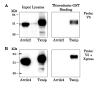
Binding of thioredoxin-GST beads to mouse Arrdc2 and Arrdc4. Epitope-tagged mouse Arrdc2, Arrdc4, and Txnip over-expressed in 293 cells. Lysates were incubated overnight with thioredoxin-C73S-GST. After washing in lysis buffer, the bound proteins were subject to Western analysis. (A) Western analysis shows that Arrdc2 was detected in the input lysate with anti-V5, but no binding to thioredoxin-GST was detected. As a positive control, under these conditions binding of mouse Txnip was successfully detected. (B) Mouse Arrdc4 was expressed with an N-terminal Xpress epitope. Input lysates and bound proteins were therefore subjected to Western analysis with anti-Xpress and anti-V5. Arrdc4 was detected in the lysate but no binding to thioredoxin was observed under conditions of successful Txnip binding. The experiments were replicated with essentially identical results as those shown.
Figure 5
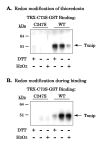
Effect of redox state on the thioredoxin-Txnip interaction. (A) Thioredoxin-C73S-GST beads were incubated in 100 μM of dithiothreitol (DTT) or hydrogen peroxide (H2O2) for two hours and washed 3 times in at least 20 bed volumes of lysis buffer. The beads were then incubated with cell lysates containing wildtype Txnip or C247S Txnip as a negative control. Bound proteins were probed for Txnip with JY2. (B) Lysates containing wildtype or C247S Txnip were added to unmodified thioredoxin-C73S-GST beads in the presence or absence of 100 μM DTT and H2O2. Bound proteins were probed for Txnip. The experiments were replicated with essentially identical results as those shown.
Figure 6
Binding of thioredoxin-GST beads to Txnip N-terminal Cys-to-Ser mutants. Wildtype and mutant Txnip proteins were overexpressed in 293 cells and the cell lysates were incubated with thioredoxin-C73S-GST. After washing, beads were incubated in sample buffer and the supernatant was probed for Txnip with JY2. Input cell lysates were probed for Txnip and demonstrated similar immunoreactivity. Similar amounts of thioredoxin-GST were observed in the supernatant by Coomassie staining. The experiment was replicated with essentially identical results as those shown.
Figure 7
Effect of Txnip on thioredoxin activity in mature adipocytes. Differentiated 3T3-L1 adipocytes were transduced with lentiviral pseudoparticles expressing human wildtype Txnip (“Txnip WT”), Txnip C247S, or an empty vector (“Empty”). After 4 days the cells were lysed and the insulin-reducing activity of thioredoxin was measured. Data were expressed as activity per total protein content and then normalized to the mean level of the empty-vector control lysates. Bars represent the mean for each group. Significant differences between groups are indicated with p-values.
Figure 8
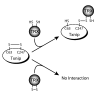
Proposed mechanism for Txnip-thioredoxin interaction. Txnip contains an intramolecular disulfide bond between C63 and C247 that allows efficient interaction with thioredoxin. Txnip forms a disulfide bond with reduced thioredoxin at C247 by disulfide exchange, yielding a stable thioredoxin mixed disulfide.
Similar articles
- The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein.
Hwang J, Suh HW, Jeon YH, Hwang E, Nguyen LT, Yeom J, Lee SG, Lee C, Kim KJ, Kang BS, Jeong JO, Oh TK, Choi I, Lee JO, Kim MH. Hwang J, et al. Nat Commun. 2014;5:2958. doi: 10.1038/ncomms3958. Nat Commun. 2014. PMID: 24389582 Free PMC article. - Txnip C247S mutation protects the heart against acute myocardial infarction.
Nakayama Y, Mukai N, Wang BF, Yang K, Patwari P, Kitsis RN, Yoshioka J. Nakayama Y, et al. J Mol Cell Cardiol. 2021 Jun;155:36-49. doi: 10.1016/j.yjmcc.2021.02.013. Epub 2021 Feb 27. J Mol Cell Cardiol. 2021. PMID: 33652022 Free PMC article. - Cardiomyocyte-specific Txnip C247S mutation improves left ventricular functional reserve in streptozotocin-induced diabetic mice.
Mukai N, Nakayama Y, Abdali SA, Yoshioka J. Mukai N, et al. Am J Physiol Heart Circ Physiol. 2021 Aug 1;321(2):H259-H274. doi: 10.1152/ajpheart.00174.2021. Epub 2021 Jun 4. Am J Physiol Heart Circ Physiol. 2021. PMID: 34085839 Free PMC article. - Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms.
Spindel ON, World C, Berk BC. Spindel ON, et al. Antioxid Redox Signal. 2012 Mar 15;16(6):587-96. doi: 10.1089/ars.2011.4137. Epub 2011 Dec 20. Antioxid Redox Signal. 2012. PMID: 21929372 Free PMC article. Review. - Physiological and Pathophysiological Roles of Thioredoxin Interacting Protein: A Perspective on Redox Inflammation and Metabolism.
Dagdeviren S, Lee RT, Wu N. Dagdeviren S, et al. Antioxid Redox Signal. 2023 Feb;38(4-6):442-460. doi: 10.1089/ars.2022.0022. Antioxid Redox Signal. 2023. PMID: 35754346 Free PMC article. Review.
Cited by
- Thioredoxin-interacting protein expression is required for VEGF-mediated angiogenic signal in endothelial cells.
Abdelsaid MA, Matragoon S, El-Remessy AB. Abdelsaid MA, et al. Antioxid Redox Signal. 2013 Dec 20;19(18):2199-212. doi: 10.1089/ars.2012.4761. Epub 2013 Jul 12. Antioxid Redox Signal. 2013. PMID: 23718729 Free PMC article. - Acute hyperglycaemia enhances oxidative stress and aggravates myocardial ischaemia/reperfusion injury: role of thioredoxin-interacting protein.
Su H, Ji L, Xing W, Zhang W, Zhou H, Qian X, Wang X, Gao F, Sun X, Zhang H. Su H, et al. J Cell Mol Med. 2013 Jan;17(1):181-91. doi: 10.1111/j.1582-4934.2012.01661.x. Epub 2013 Jan 11. J Cell Mol Med. 2013. PMID: 23305039 Free PMC article. - Induction of thioredoxin-interacting protein is mediated by oxidative stress, calcium, and glucose after brain injury in mice.
Kim GS, Jung JE, Narasimhan P, Sakata H, Chan PH. Kim GS, et al. Neurobiol Dis. 2012 May;46(2):440-9. doi: 10.1016/j.nbd.2012.02.008. Epub 2012 Feb 16. Neurobiol Dis. 2012. PMID: 22366181 Free PMC article. - Tandem ChoRE and CCAAT motifs and associated factors regulate Txnip expression in response to glucose or adenosine-containing molecules.
Yu FX, Luo Y. Yu FX, et al. PLoS One. 2009 Dec 22;4(12):e8397. doi: 10.1371/journal.pone.0008397. PLoS One. 2009. PMID: 20027290 Free PMC article. - Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases.
Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, Yodoi J. Yoshihara E, et al. Front Immunol. 2014 Jan 9;4:514. doi: 10.3389/fimmu.2013.00514. Front Immunol. 2014. PMID: 24409188 Free PMC article. Review.
References
- Griendling KK, FitzGerald GA. Circulation. 2003;108:1912–1916. - PubMed
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Science. 2004;306:457–461. - PubMed
- Henrotin Y, Kurz B, Aigner T. Osteoarthritis Cartilage. 2005;13:643–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials