Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology - PubMed (original) (raw)
doi: 10.1371/journal.pmed.0030290.
Jerome T Brooks, George M Balanos, Terence R Lappin, D Mark Layton, Dawn L Leedham, Chun Liu, Patrick H Maxwell, Mary F McMullin, Christopher J McNamara, Melanie J Percy, Christopher W Pugh, Peter J Ratcliffe, Nick P Talbot, Marilyn Treacy, Peter A Robbins
Affiliations
- PMID: 16768548
- PMCID: PMC1479389
- DOI: 10.1371/journal.pmed.0030290
Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology
Thomas G Smith et al. PLoS Med. 2006 Jul.
Abstract
Background: The von Hippel-Lindau tumour suppressor protein-hypoxia-inducible factor (VHL-HIF) pathway has attracted widespread medical interest as a transcriptional system controlling cellular responses to hypoxia, yet insights into its role in systemic human physiology remain limited. Chuvash polycythaemia has recently been defined as a new form of VHL-associated disease, distinct from the classical VHL-associated inherited cancer syndrome, in which germline homozygosity for a hypomorphic VHL allele causes a generalised abnormality in VHL-HIF signalling. Affected individuals thus provide a unique opportunity to explore the integrative physiology of this signalling pathway. This study investigated patients with Chuvash polycythaemia in order to analyse the role of the VHL-HIF pathway in systemic human cardiopulmonary physiology.
Methods and findings: Twelve participants, three with Chuvash polycythaemia and nine controls, were studied at baseline and during hypoxia. Participants breathed through a mouthpiece, and pulmonary ventilation was measured while pulmonary vascular tone was assessed echocardiographically. Individuals with Chuvash polycythaemia were found to have striking abnormalities in respiratory and pulmonary vascular regulation. Basal ventilation and pulmonary vascular tone were elevated, and ventilatory, pulmonary vasoconstrictive, and heart rate responses to acute hypoxia were greatly increased.
Conclusions: The features observed in this small group of patients with Chuvash polycythaemia are highly characteristic of those associated with acclimatisation to the hypoxia of high altitude. More generally, the phenotype associated with Chuvash polycythaemia demonstrates that VHL plays a major role in the underlying calibration and homeostasis of the respiratory and cardiovascular systems, most likely through its central role in the regulation of HIF.
Conflict of interest statement
Competing Interests: PHM, CWP, and PJR are scientific co-founders of ReOx ( http://www.reox.co.uk).
Figures
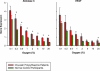
Figure 1. Aldolase C and VEGF mRNA Expression at Different Oxygen Tensions
Lymphocytes were isolated from venous blood taken from CP patients and normal control participants, and incubated at eight different levels of oxygen tension prior to RNA isolation. Gene expression is shown relative to a standard calibrator sample. Basal gene expression at 20% oxygen was significantly higher in CP patients for both_Aldolase C_ (A) and_VEGF_ (B) (p < 0.05). Both genes were induced by hypoxia, and at the lowest oxygen tension (0.1%) expression was no longer significantly different for either gene. Values are mean ± standard error of the mean. Asterisks indicate_p_ < 0.05 (unpaired_t_-test).
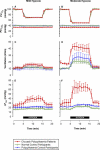
Figure 2. End-Tidal Gas Control, Ventilatory, and Pulmonary Vascular Responses to Mild and Moderate Hypoxia
(A and B) End-tidal gas control.
PetO2
and
Pet
CO2 were well controlled.
PetO2
was well matched between all groups.
Pet
CO2 was lower in the CP patient group, reflecting this group's lower baseline air-breathing
Pet
CO2. (C and D) Ventilation, given at body temperature and pressure, saturated with water vapour. Mild hypoxia provoked an increase in ventilation of 4.4 l/min in the CP patients versus 1.6 l/min in normal controls (p < 0.05), while moderate hypoxia induced increases of 24.5 versus 10.0 l/min in these two groups, respectively (p < 0.05). (E and F) Pulmonary vascular tone. This was assessed using Doppler echocardiography to determine ΔPmax, a standard non-invasive index of pulmonary vascular tone. With mild hypoxia, ΔPmax increased by 11.5 mm Hg in the CP patient group compared with only 1.1 mm Hg in the normal control group (p < 0.05). Moderate hypoxia stimulated a rise in ΔPmax of 35.3 mm Hg compared with 6.1 mm Hg in these two groups, respectively (p < 0.001). All responses are shown during mild (A, C, and E) and moderate (B, D, and F) hypoxia. Values are mean ± standard error of the mean.
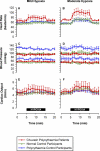
Figure 3. Systemic Vascular Responses to Mild and Moderate Hypoxia
(A and B) Heart rate. Mild hypoxia provoked a rise of 11.7 beats/min in the CP patient group, compared with 3.6 beats/min in normal control participants (p < 0.05). No other statistically significant differences in systemic vascular responses were detected between these two groups. (C and D) Blood pressure, showing systolic pressure (upper plot) and diastolic pressure (lower plot). (E and F) Cardiac output, assessed non-invasively using Doppler echocardiography. All responses are shown during mild (A, C, and E) and moderate (B, D, and F) hypoxia. Values are mean ± standard error of the mean.
Figure 4. Sensitivities to Hypoxia for Individual Participants
Results are shown in terms of the number of (normal control group) standard deviations by which each participant's response differed from the mean response of the normal control participants. The patients with CP were significantly different from the normal control group in their ventilatory and pulmonary vascular responses to both mild and moderate hypoxia, and in their heart rate responses to mild hypoxia. BP, blood pressure.
Similar articles
- Mutation of the von Hippel-Lindau gene alters human cardiopulmonary physiology.
Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, Percy MJ, Pugh CW, Ratcliffe PJ, Talbot NP, Treacy M, Robbins PA. Smith TG, et al. Adv Exp Med Biol. 2008;605:51-6. doi: 10.1007/978-0-387-73693-8_9. Adv Exp Med Biol. 2008. PMID: 18085246 - Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors.
Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, Butman JA, Jedlickova K, Prchal JT, Polyakova LA. Gordeuk VR, et al. Blood. 2004 May 15;103(10):3924-32. doi: 10.1182/blood-2003-07-2535. Epub 2004 Jan 15. Blood. 2004. PMID: 14726398 - Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2alpha gain-of-function mutation.
Formenti F, Beer PA, Croft QP, Dorrington KL, Gale DP, Lappin TR, Lucas GS, Maher ER, Maxwell PH, McMullin MF, O'Connor DF, Percy MJ, Pugh CW, Ratcliffe PJ, Smith TG, Talbot NP, Robbins PA. Formenti F, et al. FASEB J. 2011 Jun;25(6):2001-11. doi: 10.1096/fj.10-177378. Epub 2011 Mar 9. FASEB J. 2011. PMID: 21389259 Free PMC article. - The Role of VHL in the Development of von Hippel-Lindau Disease and Erythrocytosis.
Hudler P, Urbancic M. Hudler P, et al. Genes (Basel). 2022 Feb 17;13(2):362. doi: 10.3390/genes13020362. Genes (Basel). 2022. PMID: 35205407 Free PMC article. Review. - The human side of hypoxia-inducible factor.
Smith TG, Robbins PA, Ratcliffe PJ. Smith TG, et al. Br J Haematol. 2008 May;141(3):325-34. doi: 10.1111/j.1365-2141.2008.07029.x. Br J Haematol. 2008. PMID: 18410568 Free PMC article. Review.
Cited by
- Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation.
Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, Christofidou-Solomidou M, Lappin TR, Lee FS. Tan Q, et al. J Biol Chem. 2013 Jun 14;288(24):17134-44. doi: 10.1074/jbc.M112.444059. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640890 Free PMC article. - Iron, oxygen, and the pulmonary circulation.
Frise MC, Robbins PA. Frise MC, et al. J Appl Physiol (1985). 2015 Dec 15;119(12):1421-31. doi: 10.1152/japplphysiol.00179.2015. Epub 2015 Jun 11. J Appl Physiol (1985). 2015. PMID: 26066825 Free PMC article. Review. - Complex role of the HIF system in cardiovascular biology.
Czibik G. Czibik G. J Mol Med (Berl). 2010 Nov;88(11):1101-11. doi: 10.1007/s00109-010-0646-x. Epub 2010 Jun 24. J Mol Med (Berl). 2010. PMID: 20574807 Review. - High altitude adaptation in Daghestani populations from the Caucasus.
Pagani L, Ayub Q, MacArthur DG, Xue Y, Baillie JK, Chen Y, Kozarewa I, Turner DJ, Tofanelli S, Bulayeva K, Kidd K, Paoli G, Tyler-Smith C. Pagani L, et al. Hum Genet. 2012 Mar;131(3):423-33. doi: 10.1007/s00439-011-1084-8. Epub 2011 Sep 9. Hum Genet. 2012. PMID: 21904933 Free PMC article. - The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes.
Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. Pugliese SC, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Feb 1;308(3):L229-52. doi: 10.1152/ajplung.00238.2014. Epub 2014 Nov 21. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25416383 Free PMC article. Review.
References
- Semenza GL. Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. - PubMed
- Maxwell P, Wiesener M, Chang G, Clifford S, Vaux E, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. - PubMed
- Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel–Lindau protein. Nat Cell Biol. 2000;2:423–427. - PubMed
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O 2-regulated prolyl hydroxylation . Science. 2001;292:468–472. - PubMed
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, et al. HIF-α targeted for VHL-mediated destruction by proline hydroxylation: Implications for O 2 sensing . Science. 2001;292:464–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources