Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations - PubMed (original) (raw)
Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations
Conrad C Alano et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1), when activated by DNA damage, promotes both cell death and inflammation. Here we report that PARP-1 enzymatic activity is directly inhibited by minocycline and other tetracycline derivatives that have previously been shown to have neuroprotective and anti-inflammatory actions. These agents were evaluated by using cortical neuron cultures in which PARP-1 activation was induced by the genotoxic agents N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) or 3-morpholinosydnonimine (SIN-1). In both conditions, neuronal death was reduced by >80% either by 10 muM 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone, an established PARP inhibitor, or by 100 nM minocycline. Neuronal NAD(+) depletion and poly(ADP-ribose) formation, which are biochemical markers of PARP-1 activation, were also blocked by 100 nM minocycline. A direct, competitive inhibition of PARP-1 by minocycline (K(i) = 13.8 +/- 1.5 nM) was confirmed by using recombinant PARP-1 in a cell-free assay. Comparison of several tetracycline derivatives showed a strong correlation (r(2) = 0.87) between potency as a PARP-1 inhibitor and potency as a neuroprotective agent during MNNG incubations, with the rank order of potency being minocycline > doxycycline > demeclocycline > chlortetracycline. These compounds are known to have other actions that could contribute their neuroprotective effects, but at far higher concentrations than shown here to inhibit PARP-1. The neuroprotective and antiinflammatory effects of minocycline and other tetracycline derivatives may be attributable to PARP-1 inhibition in some settings.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Fig. 1.
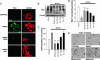
Minocycline prevents PARP-1-mediated neuronal death. (A) Photomicrographs of neuronal cultures, 24 h after 30-min incubations with MNNG (75 μM) alone or in combination with minocycline (Mino, 100 nM) or the established PARP inhibitors DPQ (25 μM) or PJ34 (100 nM). Control wells received medium exchanges only. Live cells are phase-bright with well demarcated cell bodies. (Scale bar, 20 μm.) (B) Dose–response curves of Mc, DPQ, and PJ34 on MNNG-induced neuronal death. (C) Dose–response curves of Mc, DPQ, and PJ34 on SIN-1-induced neuronal death. Neurons were incubated with 2 mM SIN-1 for 60 min. (D) The neuroprotective effects of minocycline (Mc, 100 nM) are not additive with DPQ (25 μM). ∗∗, P < 0.01 vs. all other conditions; _P_ > 0.1 for comparisons between the DPQ plus Mc condition vs. DPQ alone or vs. Mc alone. Data in B_–_D are means ± SEM of three independent experiments, each performed in triplicate.
Fig. 2.
Minocycline inhibits of PARP-1 activity in neurons. (A) Photomicrographs show immunostaining for poly(ADP-ribose) (PAR, Left), and nuclei identified in the same fields with propidium iodide (Right). Neurons were fixed for staining 15–30 min after a 10-min incubation with 75 μM MNNG. PAR formation was suppressed by the PARP inhibitors DPQ (25 μM) and PJ34 (100 nM), and by minocycline (Mc, 100 nM). (Scale bar, 20 μm.) Representative of three independent studies. (B) Western blot of PAR formation in neurons harvested 15 min after a 10-min incubation with 75 μM MNNG alone or in combination with the designated concentrations of DPQ or minocycline. Control wells received medium exchanges only. PAR is seen as a discrete, major band at 116 kDa, corresponding to PAR formation on PARP-1 itself, and a smear of other bands resulting from PAR formation on other proteins. β-actin bands indicate relative protein loading in each lane. (C) Quantification of PAR Western blots. Data are means ± SEM of three independent experiments, each performed in triplicate. ∗∗, P < 0.01 compared to MNNG alone. (D) NAD+ content in neurons harvested after 30-min incubation with 75 μM MNNG. Data are means ± SEM of three independent experiments, each performed in triplicate. ∗∗, P < 0.01 compared to MNNG alone. (E) DNA damage in neurons visualized with the PANT method, conditions as in A. The increase in PANT-labeled neurons induced by MNNG was not blocked by DPQ or Mc.
Fig. 3.
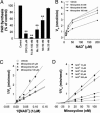
Minocycline inhibition of recombinant PARP-1. (A) Activity of isolated, recombinant PARP-1 was inhibited by DPQ, PJ34, and minocycline. Data from each of five independent experiments were normalized to the control (no inhibitor) synthetic rate. ∗∗, P < 0.01 vs. control. (B) Plot of PARP-1 enzyme activity at varying NAD+ concentrations in the presence of minocycline (25, 50, and 100 nM) or vehicle. A Lineweaver–Burke plot of these data (C) show the inhibition to be competitive with respect to NAD+, and a Dixon plot (D) shows the _K_i value to be 13.8 ± 1.5 nM.
Fig. 4.
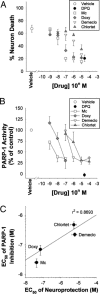
Relative potency of tetracycline derivatives as neuroprotectants and PARP-1 inhibitors. (A) Neuron death evaluated 24 h after 30-min incubations with MNNG (75 μM) alone or in combination with the designated concentrations minocycline (Mino), doxycycline (Doxy), demeclocycline (Demeclo), chlortetracyceline (Chlortet), or the established PARP inhibitor DPQ. (B) Activity of isolated, recombinant PARP-1 in the presence of the same agents used in A. (C) Scatter plot showing the relative potencies of the tetracycline derivatives as neuroprotectants and PARP-1 inhibitors. Data are means ± SEM.
Fig. 5.
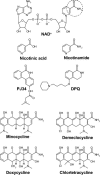
Structures of NAD+ and competitive PARP-1 inhibitors. An aromatic ring-linked carboxamide group (circled) or carbamoyl group built in a polyaromatic heterocyclic skeleton is shared by the natural PARP-1 substrate NAD+, the competitive PARP-1 inhibitors nicotinamide, DPQ, and PJ34, and the tetracycline derivatives. Nicotinic acid is not a PARP-1 inhibitor and differs from nicotinamide by the lack of the amide group.
Similar articles
- Minocycline protects cardiac myocytes against simulated ischemia–reperfusion injury by inhibiting poly(ADP-ribose) polymerase-1.
Tao R, Kim SH, Honbo N, Karliner JS, Alano CC. Tao R, et al. J Cardiovasc Pharmacol. 2010 Dec;56(6):659-68. doi: 10.1097/FJC.0b013e3181faeaf0. J Cardiovasc Pharmacol. 2010. PMID: 20881608 Free PMC article. - A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia.
Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, Ishida J, Yamamoto H, Hattori K, Matsuoka N, Mutoh S. Iwashita A, et al. J Pharmacol Exp Ther. 2004 Aug;310(2):425-36. doi: 10.1124/jpet.104.066944. Epub 2004 Apr 9. J Pharmacol Exp Ther. 2004. PMID: 15075382 - Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death.
Kauppinen TM, Swanson RA. Kauppinen TM, et al. J Immunol. 2005 Feb 15;174(4):2288-96. doi: 10.4049/jimmunol.174.4.2288. J Immunol. 2005. PMID: 15699164 - The role of poly(ADP-ribose) polymerase-1 in CNS disease.
Kauppinen TM, Swanson RA. Kauppinen TM, et al. Neuroscience. 2007 Apr 14;145(4):1267-72. doi: 10.1016/j.neuroscience.2006.09.034. Epub 2006 Nov 2. Neuroscience. 2007. PMID: 17084037 Review. - Poly(ADP-ribose) polymerase inhibitors.
Southan GJ, Szabó C. Southan GJ, et al. Curr Med Chem. 2003 Feb;10(4):321-40. doi: 10.2174/0929867033368376. Curr Med Chem. 2003. PMID: 12570705 Review.
Cited by
- Modalities and Mechanisms of Treatment for Coronavirus Disease 2019.
Zuo Z, Wu T, Pan L, Zuo C, Hu Y, Luo X, Jiang L, Xia Z, Xiao X, Liu J, Ye M, Deng M. Zuo Z, et al. Front Pharmacol. 2021 Feb 8;11:583914. doi: 10.3389/fphar.2020.583914. eCollection 2020. Front Pharmacol. 2021. PMID: 33643033 Free PMC article. Review. - Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury.
Kovesdi E, Kamnaksh A, Wingo D, Ahmed F, Grunberg NE, Long JB, Kasper CE, Agoston DV. Kovesdi E, et al. Front Neurol. 2012 Jul 16;3:111. doi: 10.3389/fneur.2012.00111. eCollection 2012. Front Neurol. 2012. PMID: 22811676 Free PMC article. - Anti-oxidative or anti-inflammatory additives reduce ischemia/reperfusions injury in an animal model of cardiopulmonary bypass.
Salameh A, Dhein S, Mewes M, Sigusch S, Kiefer P, Vollroth M, Seeger J, Dähnert I. Salameh A, et al. Saudi J Biol Sci. 2020 Jan;27(1):18-29. doi: 10.1016/j.sjbs.2019.04.003. Epub 2019 Apr 5. Saudi J Biol Sci. 2020. PMID: 31889812 Free PMC article. - Minocycline reduces neuronal death and attenuates microglial response after pediatric asphyxial cardiac arrest.
Tang M, Alexander H, Clark RS, Kochanek PM, Kagan VE, Bayir H. Tang M, et al. J Cereb Blood Flow Metab. 2010 Jan;30(1):119-29. doi: 10.1038/jcbfm.2009.194. Epub 2009 Sep 16. J Cereb Blood Flow Metab. 2010. PMID: 19756023 Free PMC article. - Treating Traumatic Brain Injury with Minocycline.
Bergold PJ, Furhang R, Lawless S. Bergold PJ, et al. Neurotherapeutics. 2023 Oct;20(6):1546-1564. doi: 10.1007/s13311-023-01426-9. Epub 2023 Sep 18. Neurotherapeutics. 2023. PMID: 37721647 Free PMC article. Review.
References
- Tikka T., Usenius T., Tenhunen M., Keinanen R., Koistinaho J. J. Neurochem. 2001;78:1409–1414. - PubMed
- Morimoto N., Shimazawa M., Yamashima T., Nagai H., Hara H. Brain Res. 2005;1044:8–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous