Excitatory and feed-forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains - PubMed (original) (raw)
Excitatory and feed-forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains
Vitaly A Klyachko et al. PLoS Biol. 2006 Jul.
Abstract
Short-term synaptic plasticity (STP) is an important mechanism for modifying neural circuits during computation. Although STP is much studied, its role in the processing of complex natural spike patterns is unknown. Here we analyze the responses of excitatory and inhibitory hippocampal synapses to natural spike trains at near-physiological temperatures. Our results show that excitatory and inhibitory synapses express complementary sets of STP components that selectively change synaptic strength during epochs of high-frequency discharge associated with hippocampal place fields. In both types of synapses, synaptic strength rapidly alternates between a near-constant level during low activity and another near-constant, but elevated (for excitatory synapses) or reduced (for inhibitory synapses) level during high-frequency epochs. These history-dependent changes in synaptic strength are largely independent of the particular temporal pattern within the discharges, and occur concomitantly in the two types of synapses. When excitatory and feed-forward inhibitory synapses are co-activated within the hippocampal feed-forward circuit unit, the net effect of their complementary STP is an additional increase in the gain of excitatory synapses during high-frequency discharges via selective disinhibition. Thus, excitatory and feed-forward inhibitory hippocampal synapses in vitro act synergistically as an adaptive filter that operates in a switch-like manner and is selective for high-frequency epochs.
Figures
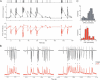
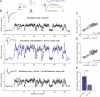
Figure 1. Excitatory and Inhibitory Synapses Act as Adaptive Filters during Natural Spike Trains
(A) The top trace illustrates a natural spike train, with each vertical line indicating an action potential in the presynaptic neuron. Relative changes in whole-cell EPSCs (middle trace; black) and IPSCs (lower trace; red) recorded from two different cells during the natural stimulation pattern shown on the top. Each point is an average of four (EPSCs) or seven (IPSCs) recordings from the same cell. Current peak values are normalized to average control values recorded at 0.1 Hz before each stimulation epoch. Four controls before and three after the train are shown, but they are plotted on a shorter timescale for clarity. (B) Three representative raw EPSC (top, black) and IPSC (bottom, red) responses (synaptic current as a function of time) to high-frequency epochs, recorded from the same cells as in (A). Responses to the first, second and sixth epochs are shown. An asterisk (*) denotes frequent cases of summation of closely spaced responses, that, in the case of IPSCs, may occasionally reach levels larger than control (see text for details). (C) Distributions of amplitude changes for data in (A). The amplitude distributions were approximated with two overlapping Gaussians centered at the peaks. Top panel (black) for EPSCs and bottom panel (red) for IPSCs.
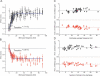
Figure 2. Changes in Excitatory and Inhibitory Synaptic Strength Occur between Two Near-Constant Levels and Are Largely Independent of the Discharge Temporal Pattern
(A and B) Excitatory (A) and inhibitory (B) gain values (from Figure 1A) are plotted as a function of the stimulus frequency in the train (1/ISI) and show a rapid transition from one gain level to another. The solid lines represent sigmoidal Boltzmann equation fits with A1 set to 1 and give the transition frequency of 7.1 and 5.6 Hz for excitatory and inhibitory gain changes, respectively. Note that as long as the stimulus is within the discharge, it is likely to result in an elevated gain level, even though its frequency might be below the gain transition range. Two such responses at approximately 1 and 2.5 Hz can be seen in (A), but are infrequent. (C and D) Average changes in excitatory (top) and inhibitory (bottom) synaptic gain during each high-frequency epoch (see Materials and Methods for definition) are plotted as a function of the average stimulus frequency within the epoch (C) and the number of stimuli in the epoch (D) for 44 epochs from five different patterns (n = 36, 14 cells). Note that the plots for excitatory and inhibitory gain changes are shown on different scales.
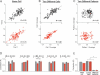
Figure 3. Filtering Patterns of Excitatory and Inhibitory Synapses Are Highly Conserved
(A) Normalized response amplitudes during two presentations of the same natural spike pattern (as in Figure 1) for one cell are plotted point-by-point against each other; the correlation coefficient is determined with linear regression. Top panel (black) is for EPSCs and bottom panel (red) for IPSCs. (B and C) Same as (A), but for the average responses from two different cells to the same (B) or to two different (C) stimulation patterns. (D) Top are representations of five different natural spike trains used. Bar graphs below give average correlation values determined as shown in (A–C) for the five different spike patterns. For each pattern, correlations between two presentations for one cell (two left bars,n = 4–11 cells/pattern) and between average responses from two different cells (two right bars,n = 6–11 random cell pairs/pattern) are plotted for excitatory (black) and inhibitory (red) currents respectively. (E) Summary of the data from (D). ** indicates_p_ < 10−6.
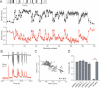
Figure 4. Gain Changes in Excitatory and Inhibitory Synapses Are Simultaneous and (Anti)Correlated
(A) Excitatory (top, black) and inhibitory (bottom, red) normalized response amplitudes for a natural spike train (different than that shown in Figure 1) are plotted versus stimulus number within the train. Each point is an average of four (EPSCs) or seven (IPSCs) recordings from the same cells. Four control responses, before and after the spike train, are obtained by stimulation at a constant rate of 0.1 Hz. (B) Representative raw EPSC (top, black) and IPSC (bottom, red) responses (synaptic current as a function of time) to a high-frequency epoch, recorded from the same cells as in (A). (C) IPSC and EPSC amplitudes during the train from (A) are plotted versus each other and the correlation is determined as described in Figures 3A– 3C. (D) Average correlation between changes in EPSC and IPSC amplitudes for five different spike patterns. Ten random pairs of cells are selected for each pattern. ** indicates_p_ < 10−18.
Figure 5. Complementary Expression of STP Components Underlies Opposite Gain Changes of Excitatory and Inhibitory Synapses
(A) Top is a representation of the spike train used. Below are whole-cell recordings (current as a function of time) of IPSCs (middle, red) and EPSCs (bottom, black) from two different cells in response to a 150-stimulus, 20 Hz train. First six and 150th responses are shown. (B) Average excitatory (upward going, black) and inhibitory (downward going, red) response amplitudes for 150-stimulus trains at 2, 10, 20, and 40 Hz. Average EPSC response at 20 Hz falls between those at 10 Hz and 40 Hz and is omitted for clarity. Each point is an average of_n_ = 9–17 cells for EPSCs and_n_ = 5–9 cells for IPSCs. (C) Paired-pulse plasticity, measured at intervals 20 ms to 2 s, for excitatory (black) and inhibitory (red) synapses. Inset. Representative excitatory (top, black) and inhibitory (bottom, red) paired responses (current as a function of time) at a 50-ms interval. (D) A mixture of augmentation and the very slow component of depression is unmasked 5 s after the end of 150 stimuli at 40 Hz. Augmentation was extracted from the mixture using the average monoexponential fit parameters of the very slow depression (τ = 49 ± 9 s,n = 13) and assuming a multiplicative relationship between these components [ 44, 45, 58] (inset). (E) Rapid component of depression was studied with the test pulses applied at different intervals (250 ms to 6 s) after 150 stimuli at 40 Hz for excitatory (black) and inhibitory (red) synapses. For EPSCs, recovery from depression was contaminated mostly by the decaying facilitation and augmentation. Rapid component of depression was isolated from the mixture using the analysis introduced by Magleby and Zengel [ 44] and further developed for the hippocampal synapses [ 58], assuming multiplicative relationship among the components and using monoexponential fit parameters of facilitation and augmentation determined for each cell as described in (C) and (D).
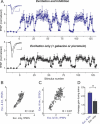
Figure 6. Feed-Forward Inhibition Provides Additional Gain for Excitatory Transmission Selectively during High-Frequency Epochs
(A) A schematic of a basic disynaptic feed-forward circuit unit in the hippocampal CA3-CA1 areas (left panel). Pure excitatory responses (Exc.) were isolated by holding the cell membrane near the Cl− reversal potential (−84 mV) in the absence of AMPA/GABAA receptor antagonists (black sample whole-cell recording trace in the right panel). EPSC/IPSC sequence (Exc. & Inh.) was recorded in the same cell by changing the holding potential to −54 mV (blue sample trace in the right panel). EPSC/IPSC sequence was completely blocked by the AMPA receptor antagonist DNQX (orange sample trace in the right panel), demonstrating the absence of direct stimulation of local inhibitory fibers. Scale bars indicate 10 ms and 100 pA. (B) Top panel: pure excitatory responses to natural stimulus train (same train as in Figure 1) recorded at −84 mV from a different cell than that in (A). The average gain during the train was 1.61 ± 0.08,n = 9. The average gain during natural stimulation was significantly larger at −54 mV (middle panel), where excitation and inhibition were present together (p < 0.005,n_ = 9). This additional gain increase was completely blocked at −54 mV by GABAA receptor antagonist gabazine (p > 0.9, bottom panel). Note that in none of these recordings did we subtract contributions from the preceding currents. All recordings shown were from the same cell, and each point was an average of four responses. Each dataset was normalized to its own control. Voltage measurements were a posteriori corrected for the liquid junction potential (see Materials and Methods). Insets: Representative single traces of control current and current with increased gain during one of the discharges are shown for each panel. Scale bars indicate 10 ms and 100 pA. (C) Correlation of response amplitudes from (B) recorded at −84 mV (Excitation only) and at −54 mV (Excitation + Inhibition). (D) Correlation of response amplitudes from (B) recorded at −54 mV after gabazine application (Excitation only) and before gabazine application (Excitation + Inhibition). (E) Average gain during natural spike trains (all points included) for excitation alone (Exc. only) or in the presence of inhibition (Exc. & Inh.);n = 9 cells, two different patterns. ** indicates_p < 0.005.
Figure 7. Synergistic Action of Excitation and Feed-Forward Inhibition Is Observed in the Intact Hippocampal Feed-Forward Circuit Unit
(A) fPSPs were recorded in hippocampal slices in response to the same natural spike pattern as in Figure 6, in the presence of inhibition (top) or with inhibition blocked by gabazine or picrotoxin (bottom). All recordings shown were from the same slice, and each point was an average of four responses. Insets: Representative single traces of control fPSP and fPSP with increased gain during one of the discharges are shown for each panel. The downward part of the stimulus artifact was removed for clarity. Scale bars indicate 10 ms and 0.3 mV. (B) Correlation of fPSP responses from (A) recorded before and after gabazine application (Excitation + Inhibition vs. Excitation only). (C) Correlation of fPSP and EPSC responses recorded in the presence of inhibition. (D) Average gain of fPSPs (all points included) during natural spike trains for excitation alone (Exc.only) or in the presence of inhibition (Exc. & Inh.);n = 7 slices, four different patterns. ** indicates_p_ < 0.01.
Comment in
- Neurons' short-term plasticity amplifies signals.
Inman M. Inman M. PLoS Biol. 2006 Jul;4(7):e240. doi: 10.1371/journal.pbio.0040240. Epub 2006 Jun 20. PLoS Biol. 2006. PMID: 20076608 Free PMC article. No abstract available.
Similar articles
- Dynamic balance of excitation and inhibition rapidly modulates spike probability and precision in feed-forward hippocampal circuits.
Wahlstrom-Helgren S, Klyachko VA. Wahlstrom-Helgren S, et al. J Neurophysiol. 2016 Dec 1;116(6):2564-2575. doi: 10.1152/jn.00413.2016. Epub 2016 Sep 7. J Neurophysiol. 2016. PMID: 27605532 Free PMC article. - Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses.
Klyachko VA, Stevens CF. Klyachko VA, et al. J Neurosci. 2006 Jun 28;26(26):6945-57. doi: 10.1523/JNEUROSCI.1382-06.2006. J Neurosci. 2006. PMID: 16807324 Free PMC article. - Differences in multiple forms of short-term plasticity between excitatory and inhibitory hippocampal neurons in culture.
Kaplan MP, Wilcox KS, Dichter MA. Kaplan MP, et al. Synapse. 2003 Oct;50(1):41-52. doi: 10.1002/syn.10244. Synapse. 2003. PMID: 12872293 - Excitatory synaptic integration in hippocampal pyramids and dentate granule cells.
Andersen P, Raastad M, Storm JF. Andersen P, et al. Cold Spring Harb Symp Quant Biol. 1990;55:81-6. doi: 10.1101/sqb.1990.055.01.010. Cold Spring Harb Symp Quant Biol. 1990. PMID: 2132857 Review. No abstract available. - Synapse-specific homeostatic mechanisms in the hippocampus.
Deeg KE. Deeg KE. J Neurophysiol. 2009 Feb;101(2):503-6. doi: 10.1152/jn.91115.2008. Epub 2008 Dec 10. J Neurophysiol. 2009. PMID: 19073801 Free PMC article. Review.
Cited by
- A Slow Short-Term Depression at Purkinje to Deep Cerebellar Nuclear Neuron Synapses Supports Gain-Control and Linear Encoding over Second-Long Time Windows.
Pedroarena CM. Pedroarena CM. J Neurosci. 2020 Jul 29;40(31):5937-5953. doi: 10.1523/JNEUROSCI.2078-19.2020. Epub 2020 Jun 17. J Neurosci. 2020. PMID: 32554551 Free PMC article. - The Mechanisms and Functions of Synaptic Facilitation.
Jackman SL, Regehr WG. Jackman SL, et al. Neuron. 2017 May 3;94(3):447-464. doi: 10.1016/j.neuron.2017.02.047. Neuron. 2017. PMID: 28472650 Free PMC article. Review. - Predictable Fluctuations in Excitatory Synaptic Strength Due to Natural Variation in Presynaptic Firing Rate.
Ren N, Wei G, Ghanbari A, Stevenson IH. Ren N, et al. J Neurosci. 2022 Nov 16;42(46):8608-8620. doi: 10.1523/JNEUROSCI.0808-22.2022. Epub 2022 Sep 28. J Neurosci. 2022. PMID: 36171085 Free PMC article. - How do short-term changes at synapses fine-tune information processing?
Klug A, Borst JG, Carlson BA, Kopp-Scheinpflug C, Klyachko VA, Xu-Friedman MA. Klug A, et al. J Neurosci. 2012 Oct 10;32(41):14058-63. doi: 10.1523/JNEUROSCI.3348-12.2012. J Neurosci. 2012. PMID: 23055473 Free PMC article. Review. - Target-cell-specific Short-term Plasticity Reduces the Excitatory Drive onto CA1 Interneurons Relative to Pyramidal Cells During Physiologically-derived Spike Trains.
Sun HY, Li Q, Bartley AF, Dobrunz LE. Sun HY, et al. Neuroscience. 2018 Sep 15;388:430-447. doi: 10.1016/j.neuroscience.2018.07.051. Epub 2018 Aug 10. Neuroscience. 2018. PMID: 30099117 Free PMC article.
References
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. - PubMed
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources