Functional imaging reveals numerous fields in the monkey auditory cortex - PubMed (original) (raw)
Functional imaging reveals numerous fields in the monkey auditory cortex
Christopher I Petkov et al. PLoS Biol. 2006 Jul.
Abstract
Anatomical studies propose that the primate auditory cortex contains more fields than have actually been functionally confirmed or described. Spatially resolved functional magnetic resonance imaging (fMRI) with carefully designed acoustical stimulation could be ideally suited to extend our understanding of the processing within these fields. However, after numerous experiments in humans, many auditory fields remain poorly characterized. Imaging the macaque monkey is of particular interest as these species have a richer set of anatomical and neurophysiological data to clarify the source of the imaged activity. We functionally mapped the auditory cortex of behaving and of anesthetized macaque monkeys with high resolution fMRI. By optimizing our imaging and stimulation procedures, we obtained robust activity throughout auditory cortex using tonal and band-passed noise sounds. Then, by varying the frequency content of the sounds, spatially specific activity patterns were observed over this region. As a result, the activity patterns could be assigned to many auditory cortical fields, including those whose functional properties were previously undescribed. The results provide an extensive functional tessellation of the macaque auditory cortex and suggest that 11 fields contain neurons tuned for the frequency of sounds. This study provides functional support for a model where three fields in primary auditory cortex are surrounded by eight neighboring "belt" fields in non-primary auditory cortex. The findings can now guide neurophysiological recordings in the monkey to expand our understanding of the processing within these fields. Additionally, this work will improve fMRI investigations of the human auditory cortex.
Figures
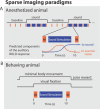
Figure 1. Sparse-Imaging Paradigms
(A) The sparse-imaging paradigm for imaging the anesthetized animals (at 4.7- and 7-T) consisted of a block design with alternating stimulation and baseline periods. Data acquisition and sound stimulus were interleaved as schematized in the blow-out of a single sequence during a stimulation block. Here, the sequence initiates with an imaged brain volume, allowing sounds to be presented during the subsequent silent period. Because of the delay in the hemodynamic (BOLD) response the next volume acquired 10 s later reflects the sound stimulation that occurred approximately 6 s before. This minimizes influences on the auditory cortex BOLD response by the scanner noise that occurred 10 s before. This sequence is repeated four times during a stimulation block and four times during a baseline (no-stimulation) block. (B) For imaging the behaving animal (at 7-T), the animal completed behavioral trials composed of the single sparse-imaging sequence during minimal eye and body movements (see “Behaving Animal Preparation and Imaging” in Materials and Methods).
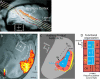
Figure 2. Macaque fMR Imaging and ACFs
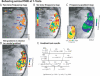
(A) Imaging slices were aligned with the lateral sulcus to obtain a plane of auditory cortex activity on the lower bank of this sulcus (colored in translucent orange; STS, superior temporal sulcus). (B) Shows activations to broadband noise from one anesthetized animal. (C) Schematizes the possible ACFs contributing to activity in (B) [ , , – 21]. (D) Schematizes functional properties of ACFs that could distinguish them. Two of the three core or primary ACFs (blue) have frequency selective gradients, shown with arrows from low (L) to high (H) frequency selectivity. The question mark in the core field RT indicates limited evidence of such a gradient in primates [ 2]. Anatomy suggests there could be seven or eight belt or non-primary ACFs (orange) [ 1, 3]. Only four of the belt ACFs have neurophysiologically described frequency gradients, and these fields are considered to be less responsive to single-frequency tones, preferring instead more complex sounds [ 16, 18, 19, 21, 28, 29]. Abbreviations: Ec, external capsule; Cis, circular sulcus.
Figure 3. Two Tones Reveal Multiple Frequency Selective Regions over the Auditory Core
(A) An experiment with alternating blocks of 0.5 kHz (Low) and 8 kHz (High) frequency tones. First, significantly active voxels to these sounds are identified (see inset). Within this region, individual voxels' frequency selectivity is determined by the difference in the signal correlation to the Low (red to yellow) vs. High (blue to cyan) tones. The results are thresholded to not display weakly frequency selective voxels. Four frequency selective regions are observed: (H1) labels a posterior high-frequency selective region, (L1) a more anterior low-frequency region that could be shared by the anterior portion of A1 and the posterior of R (see schematic of the auditory core to right), (H2) an anterior high-frequency selective region, shared by R and RT, and (L2) the most anterior low-frequency selective region observed, belonging to field RT (also see Figure 2D). (B) Timecourse of the BOLD response illustrating that clusters of voxels underneath the labels in (A) showed greater activation to the high frequency tone at H1 and H2 but greater activation to the low frequency tone at L1 and L2 (see ** during sound stimulation periods;t_-tests, all_p < 0.002). Error-bars signify standard error of the mean across stimulation block repeats.
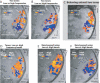
Figure 4. Reliable Frequency-Selective Regions in the Behaving and in the Anesthetized Animals
Observed throughout these examples are the two pairs of high and low frequency selective regions as shown in Figure 3, i.e., H1, L1, H2, and L2. (A–C) the results from using two tones. (A–B) show different scanning days with the same anesthetized animal as in Figure 5 (J02, the animal and the experiment are identified in the lower right of each panel). Arrow in (A) shows the axis along which the frequency selective regions lie. (C) Behaving animal results (animal J03). (D–F) Further results with anesthetized animals (J02 and E02). (D) Results using two combinations of tone frequencies (Low: 0.5, 1, and 2 kH tones; High: 4, 8, and 16 kHz tones). (E–F) In two animals these panels reveal the patterns of activity for low and high frequency one-octave band-passed noise (Low: 0.5–1 kHz; High: 4–8 kHz).
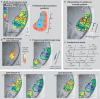
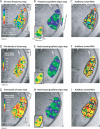
Figure 5. Delineating Borders and ROIs over Auditory Cortex
(A) Multiple tone-frequency map, resulting from the presentation of six tone frequencies individually in pseudo-randomized stimulation blocks. The best-tone frequency response of the significantly active voxels is color-coded (see “MRI data analysis” in Materials and Methods). Tone activations were extensive over auditory cortex, see text. Right of (A) schematizes the ACFs that could be active in this broad region including the directions (from Low to High) of the expected frequency selective gradients. (B) The core preferentially responds to tones in comparison to belt regions [ 19, 21]. An “auditory core” ROI was drawn around the average tone response, thresholded to the approximate length (˜1.8 cm) and full width (˜0.8 cm) of the core [ 3]. (C) The results of a gradient analysis that assigns a sign to gradients progressing in the anterior direction, along an axis predetermined by points underneath labels H1 and L2 from the data in Figure 3A. Along this axis, gradients that progress anteriorly from low to high frequencies, ascending gradients, are assigned a positive sign (green) and those from high to low, descending gradients, a negative sign (blue). Multiple mirror reversals of frequency gradients are observed and borders between ACFs are delineated where signs reverse, or the two colors meet. The smoothed version of the multi-frequency map (A) used for the gradient analysis is inset in (C). The area of the “auditory core” ROI from (B) is overlaid in transparent white over the map in (C). (D) Auditory cortex ROIs were delineated relying on the gradient sign map borders, shown here overlaid on the data from the multi-tone frequency map. Each ROI's gradient from the multi-tone data was tested in relation to a model gradient progressing from low to high values anteriorly, see colorbar. (E) Reports the results of the ROI gradient tests as correlation coefficients,r, and associated_p_-values. The combined analyses localize many auditory fields with mirror reversed frequency gradients and functionally tessellate large portions of auditory cortex. (F–I) Split datasets from the entire experiment. Format as for panels (A and C).
Figure 6. Functional Tessellation of the Behaving Animal's Auditory Cortex
(A) Contrasting responses to two tones reveals an extended alternating pattern of (Low, High, Low, High, Low) frequency selectivity. (B) Multi-tone frequency map. (C) Gradient analysis. (D) Delineated auditory cortex ROIs. (E) Statistical results of the ROI gradient tests; format as for Figure 5 (A), (C–E). Note that the_(n)_ for these data reflect the number of correctly completed trials, whereas for the anesthetized animal imaging one stimulation block contains four sparse-imaging sequences, or such “trials.”
Figure 7. Delineating ACF Borders with Multiple Tones and Bands of Noise
(A–C) An additional multi-tone experiment with animal J02. Format as for Figure 5 (A), (C and D). (D–I) Show the results from two animals where five one-octave bands of noise (band-passed noise) were used to localize ACFs. (D and G) Five best noise-band maps showing the antero-posterior frequency gradients. (E and H) show the gradient sign procedure revealing borders between neighboring ACFs. (C, F, and I) show auditory cortex ROI outlines. The results of the gradient tests within these outlines in comparison to the model gradient are shown in Table 1.
Figure 8. Comparing Noise to Tone Responses Reveals a Preference for Noise in the Belt
In nine experiments we individually presented single frequency tones within the range of the comparison band-passed noise (see “Sound stimuli and presentation” in Materials and Methods) and compared responses. (A) Shows distributions of the auditory cortex response (see “MRI data analysis” in Materials and Methods) to tone or noise stimulation. The analysis is based on voxels significantly active to at least one of the stimulation conditions, i.e., under tone or noise stimulation. Voxels responded more to noise than tones (Wilcoxon rank sum test of the distributions,p < 0.0001). (B) Noise responses were larger than tone responses in regions of the auditory belt, as illustrated by this example experiment, confirming and extending predictions from neurophysiological recordings of the lateral belt [ 19, 21].
Comment in
- The tessellated monkey: parsing the functional fields of the auditory cortex.
Gross L. Gross L. PLoS Biol. 2006 Jul;4(7):e245. doi: 10.1371/journal.pbio.0040245. Epub 2006 Jun 20. PLoS Biol. 2006. PMID: 20076612 Free PMC article. No abstract available.
Similar articles
- Spatial processing in the auditory cortex of the macaque monkey.
Recanzone GH. Recanzone GH. Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):11829-35. doi: 10.1073/pnas.97.22.11829. Proc Natl Acad Sci U S A. 2000. PMID: 11050216 Free PMC article. - Functional magnetic resonance imaging of auditory cortical fields in awake marmosets.
Toarmino CR, Yen CCC, Papoti D, Bock NA, Leopold DA, Miller CT, Silva AC. Toarmino CR, et al. Neuroimage. 2017 Nov 15;162:86-92. doi: 10.1016/j.neuroimage.2017.08.052. Epub 2017 Aug 19. Neuroimage. 2017. PMID: 28830766 Free PMC article. - Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey.
Woods TM, Lopez SE, Long JH, Rahman JE, Recanzone GH. Woods TM, et al. J Neurophysiol. 2006 Dec;96(6):3323-37. doi: 10.1152/jn.00392.2006. Epub 2006 Aug 30. J Neurophysiol. 2006. PMID: 16943318 - Functional imaging of human auditory cortex.
Woods DL, Alain C. Woods DL, et al. Curr Opin Otolaryngol Head Neck Surg. 2009 Oct;17(5):407-11. doi: 10.1097/MOO.0b013e3283303330. Curr Opin Otolaryngol Head Neck Surg. 2009. PMID: 19633556 Review. - Multisensory interactions in primate auditory cortex: fMRI and electrophysiology.
Kayser C, Petkov CI, Logothetis NK. Kayser C, et al. Hear Res. 2009 Dec;258(1-2):80-8. doi: 10.1016/j.heares.2009.02.011. Epub 2009 Mar 6. Hear Res. 2009. PMID: 19269312 Review.
Cited by
- Sound-identity processing in early areas of the auditory ventral stream in the macaque.
Kuśmierek P, Ortiz M, Rauschecker JP. Kuśmierek P, et al. J Neurophysiol. 2012 Feb;107(4):1123-41. doi: 10.1152/jn.00793.2011. Epub 2011 Nov 30. J Neurophysiol. 2012. PMID: 22131372 Free PMC article. - Delineation of a frequency-organized region isolated from the mouse primary auditory cortex.
Tsukano H, Horie M, Bo T, Uchimura A, Hishida R, Kudoh M, Takahashi K, Takebayashi H, Shibuki K. Tsukano H, et al. J Neurophysiol. 2015 Apr 1;113(7):2900-20. doi: 10.1152/jn.00932.2014. Epub 2015 Feb 18. J Neurophysiol. 2015. PMID: 25695649 Free PMC article. - Direct Relay Pathways from Lemniscal Auditory Thalamus to Secondary Auditory Field in Mice.
Ohga S, Tsukano H, Horie M, Terashima H, Nishio N, Kubota Y, Takahashi K, Hishida R, Takebayashi H, Shibuki K. Ohga S, et al. Cereb Cortex. 2018 Dec 1;28(12):4424-4439. doi: 10.1093/cercor/bhy234. Cereb Cortex. 2018. PMID: 30272122 Free PMC article. - Characterisation of the BOLD response time course at different levels of the auditory pathway in non-human primates.
Baumann S, Griffiths TD, Rees A, Hunter D, Sun L, Thiele A. Baumann S, et al. Neuroimage. 2010 Apr 15;50(3):1099-108. doi: 10.1016/j.neuroimage.2009.12.103. Epub 2010 Jan 4. Neuroimage. 2010. PMID: 20053384 Free PMC article. - Functional maps of human auditory cortex: effects of acoustic features and attention.
Woods DL, Stecker GC, Rinne T, Herron TJ, Cate AD, Yund EW, Liao I, Kang X. Woods DL, et al. PLoS One. 2009;4(4):e5183. doi: 10.1371/journal.pone.0005183. Epub 2009 Apr 13. PLoS One. 2009. PMID: 19365552 Free PMC article.
References
- Morel A, Kaas JH. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol. 1992;318:27–63. - PubMed
- Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998;394:475–495. - PubMed
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. - PubMed
- Rivier F, Clarke S. Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: Evidence for multiple auditory areas. Neuroimage. 1997;6:288–304. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials