HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain - PubMed (original) (raw)
Comparative Study
HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain
Jung-Bin Kim et al. J Neurosci. 2006.
Abstract
Cerebral ischemic injury proceeds with excitotoxicity-induced acute neuronal death in the ischemic core and with delayed damage processes in the penumbra. However, knowledge concerning the direct mediators that connect these two processes is limited. Here, we demonstrate that high-mobility group box 1 (HMGB1), a nonhistone DNA-binding protein, is massively released into the extracellular space immediately after ischemic insult and that it subsequently induces neuroinflammation in the postischemic brain. Short hairpin (sh)RNA-mediated HMGB1 downregulation in the postischemic brain suppressed infarct size, microglia activation, and proinflammatory marker induction, indicating that HMGB1 plays a crucial role in the inflammatory process. The proinflammatory cytokine-like function of extracellular HMGB1 was further verified in primary cortical cultures and microglial cultures. HMGB1 was found to accumulate in NMDA-treated primary cortical culture media, and supernatants collected from these cultures were found to trigger microglia activation, the hallmark of brain inflammation. Moreover, treatment with recombinant HMGB1 also induced microglial activation, but HMGB1-depleted supernatant produced by anti-HMGB1 antibody treatment or by HMGB1 shRNA expression did not, thus demonstrating the essential role of HMGB1 in microglial activation. Together, these results indicate that HMGB1 functions as a novel proinflammatory cytokine-like factor that connects excitotoxicity-induced acute damage processes and delayed inflammatory processes in the postischemic brain.
Figures
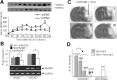
Figure 1.
Plasma and CSF HMGB1 immediately increased after MCAO. A, TTC staining was performed with coronal brain sections, which were obtained 2 d after sham operation or after 1 h of MCAO. B, HMGB1 levels in ischemic hemispheres (indicated region in bottom panel in A) were determined by immunoblotting at various times after 1 h of MCAO. C, D, HMGB1 levels in serum (C) and in CSF (D) were examined by immunoblotting. E, Total protein extracts obtained from normal brain tissue or from CSF after MCAO were loaded onto two-dimensional gels, blotted, and immunodetected with anti-HMGB1 antibody. Data are presented as means ± SEM (n = 3). *p < 0.05.
Figure 2.
shRNA-mediated silencing of HMGB1 gene expression in the normal brain. A, Schematic diagram of the HMGB1 shRNA transgene showing its sense and antisense regions and the predicted transcript, a hairpin structure with a 7 bp loop. shHMGB1-pU6 and MshHMGB1-pU6 represent wild-type and mutant shRNA (six-nucleotide substitution in red)-expressing plasmid, respectively. siRNA, Small interfering RNA. B, β-Galactosidase staining was performed 2 d after _lacZ_-expressing plasmid transfection using PAMAM-Arg as a gene carrier (left), and the boundary of the region expressing the exogenous gene was indicated (right, white box). shHMGB1-pU6 or MshHMGB1-pU6 was injected into the indicated region, and biochemical assays were done on samples prepared from the indicated area in the right panel. C, Northern blotting for shHMGB1 transcripts in shHMGB1-pU6-transfected brains was performed at the indicated times. Each lane contained total RNA obtained from five animals, and blots were probed with the 32P-labeled 64 nucleotide sense oligonucleotide. Con, Control. D, The levels of HMGB1 expression in shHMGB1-pU6- or MshHMGB1-pU6-administered animals were determined by RT-PCR at the indicated times after transfection. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. Data are presented as means ± SEM (n = 4). *p < 0.05.
Figure 3.
Neuroprotection by the shRNA-mediated silencing of HMGB1 induction in the postischemic brain. shHMGB1-pU6/PAMAM-Arg or MshHMGB1-pU6/PAMAM-Arg complexes were administered into the indicated region (C) 24 h before 1 h of MCAO. A, The serum levels of HMGB1 in shHMGB1-pU6-administered animals were determined by immunoblotting at the indicated times after 1 h of MCAO. B, The levels of HMGB1 in the brain tissues (Fig. 2_B_, right, white box) of shHMGB1-pU6- or MshHMGB1-pU6-administered animals were determined by RT-PCR 24 h after 1 h of MCAO. C, A TTC-stained infarction area in a coronal brain section 2 d after1 h of MCAO. The arrow indicates the administration point of the shHMGB1-pU6/PAMAM-Arg complex 24 h before 1 h of MCAO. Representative pictures showing suppressed infarct formation (types I and II) and no suppression (type III) are presented. D, Numbers of rescued or nonrescued animals are presented in the bar graph. Data are presented as means ± SEM (n = 3). *p < 0.05.
Figure 4.
Suppression of inflammatory markers by HMGB1 shRNA in the postischemic brain. A–D, Microglia activation 4 d after 1 h of MCAO was visualized by anti-GSA I-B4 immunostaining (large rectangle area in the inset in A). shHMGB1-pU6 (C) or MshHMGB1-pU6 (D) was administered 24 h before MCAO. Activated microglia were detected in the ischemic core (B). Activated microglia numbers were notably reduced in wild-type shRNA-expressing brains (C) but not in mutant shRNA-expressing brains (D). The insets in A–D are high-magnification micrographs. E, The numbers of GSA I-B4-positive cells in the indicated areas (0.2 × 0.3 mm) in B were obtained by scoring in a blind manner the GSA I-B4-positive cells in 12 photographs taken from three independent experiments. F, Activation of p38 MAPK was examined 24 h after 1 h of MCAO by immunoblotting using anti-phosphorylated p38 MAPK antibody. G–K, The expressions of proinflammatory markers in the presence of shHMGB1-pU6 or MshHMGB1-pU6 were examined 1 d after 1 h of MCAO. Solid bars, Sham; open bars, MCAO (H–K). Data are presented as means ± SEM (n = 3). *p < 0.05. Scale bar: A–D, 500 μm. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
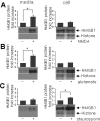
Figure 5.
Accumulation of HMGB1 in bath medium containing primary cortical cell cultures undergoing excitotoxicity-induced cell death. Primary cortical cultures were incubated in serum-free MEM containing 30 μ
m
NMDA (A), 50 μ
m
glutamate (B), or 10 μ
m
staurosporin (C) for 1 h. After 24 h, both culture media and cell homogenates were analyzed by immunoblotting with anti-HMGB1 or anti-histone antibody (A–C). Data are presented as means ± SEM (n = 3). *p < 0.05.
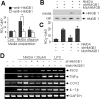
Figure 6.
Microglial activation by extracellular HMGB1. A, Primary microglial cultures (1 × 104 cells) were incubated with media collected from NMDA- or staurosporine (stauros)-treated primary cortical cultures for 24 h in the presence or absence of anti-HMGB1 antibody, and NO production was determined using the Griess method. shHMGB1-pU6 or MshHMGB1-pU6 was transiently transfected into primary cortical cells 24 h before NMDA treatment. Con, Control. B, The amounts of HMGB1 in NMDA-treated cells and in medium bathing were determined by RT-PCR after 24 h of NMDA treatment. C, Primary microglia cultures were treated with media collected from NMDA-treated primary cortical cultures, transfected or not transfected with shRNA-expressing plasmid or mutant shRNA-expressing plasmid, and NO production was determined using the Griess method. D, Changes in the RNA levels of the proinflammatory markers TNF-α, COX-2, and IL-1β in the presence of HMGB1 shRNA-expressing plasmid or of mutant shRNA-expressing plasmid were followed by RT-PCR. LPS (100 ng/ml) treatment was used as a positive control. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. The results of four independent experiments are presented as means ± SEM.
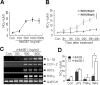
Figure 7.
Induction of microglia activation by recombinant HMGB1. A, B, Primary microglia cultures were treated with the indicated concentrations of rHMGB1 for 48 h (A) or treated with 100 or 500 ng of rHMGB1 for the indicated times (B), and NO production was determined by measuring nitrite in medium using the Griess method. C, Changes in the RNA levels of the pro-inflammatory markers iNOS, TNF-α, COX-2, and IL-1β in the presence of increasing amounts of rHMGB1 were followed by RT-PCR. D, Primary microglia cultures were treated with rHMGB1 (100 ng/ml), LPS (100 ng/ml), TNFα (50 ng/ml), or IFNγ (25 ng/ml) individually or in combination for 48 h, and NO production was determined. The data of four independent experiments are presented as means ± SEM. *p < 0.05. Con, Control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Similar articles
- Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway.
Kim SW, Lim CM, Kim JB, Shin JH, Lee S, Lee M, Lee JK. Kim SW, et al. Neurotox Res. 2011 Aug;20(2):159-69. doi: 10.1007/s12640-010-9231-x. Epub 2010 Nov 30. Neurotox Res. 2011. PMID: 21116767 - Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain.
Kim JB, Lim CM, Yu YM, Lee JK. Kim JB, et al. J Neurosci Res. 2008 Apr;86(5):1125-31. doi: 10.1002/jnr.21555. J Neurosci Res. 2008. PMID: 17975839 - Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion.
Kim SW, Jin Y, Shin JH, Kim ID, Lee HK, Park S, Han PL, Lee JK. Kim SW, et al. Neurobiol Dis. 2012 Apr;46(1):147-56. doi: 10.1016/j.nbd.2011.12.056. Epub 2012 Jan 13. Neurobiol Dis. 2012. PMID: 22266336 - Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia.
Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. Yang QW, et al. Front Biosci (Schol Ed). 2010 Jun 1;2(3):1081-91. doi: 10.2741/s119. Front Biosci (Schol Ed). 2010. PMID: 20515842 Review. - Neurons Are a Primary Driver of Inflammation via Release of HMGB1.
Yang H, Andersson U, Brines M. Yang H, et al. Cells. 2021 Oct 18;10(10):2791. doi: 10.3390/cells10102791. Cells. 2021. PMID: 34685772 Free PMC article. Review.
Cited by
- Immune players in the CNS: the astrocyte.
Jensen CJ, Massie A, De Keyser J. Jensen CJ, et al. J Neuroimmune Pharmacol. 2013 Sep;8(4):824-39. doi: 10.1007/s11481-013-9480-6. Epub 2013 Jul 4. J Neuroimmune Pharmacol. 2013. PMID: 23821340 Review. - PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice.
Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W, Yang Q, Qi J, Zhou J, Wang J. Wu H, et al. Neurobiol Aging. 2015 Mar;36(3):1439-50. doi: 10.1016/j.neurobiolaging.2014.12.029. Epub 2015 Jan 3. Neurobiol Aging. 2015. PMID: 25623334 Free PMC article. - Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming.
Frank MG, Weber MD, Watkins LR, Maier SF. Frank MG, et al. Brain Behav Immun. 2015 Aug;48:1-7. doi: 10.1016/j.bbi.2015.03.010. Epub 2015 Mar 24. Brain Behav Immun. 2015. PMID: 25816800 Free PMC article. Review. - Hypothermia inhibits the propagation of acute ischemic injury by inhibiting HMGB1.
Lee JH, Yoon EJ, Seo J, Kavoussi A, Chung YE, Chung SP, Park I, Kim CH, You JS. Lee JH, et al. Mol Brain. 2016 Aug 20;9(1):81. doi: 10.1186/s13041-016-0260-0. Mol Brain. 2016. PMID: 27544687 Free PMC article. - Acetylcholinesterase loosens the brain's cholinergic anti-inflammatory response and promotes epileptogenesis.
Gnatek Y, Zimmerman G, Goll Y, Najami N, Soreq H, Friedman A. Gnatek Y, et al. Front Mol Neurosci. 2012 May 18;5:66. doi: 10.3389/fnmol.2012.00066. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22639569 Free PMC article.
References
- Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995). Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke 26:1478–1489. - PubMed
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ (2000). HMG-1 as a mediator of acute lung inflammation. J Immunol 165:2950–2954. - PubMed
- Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P (2002). HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine 18:231–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources